Benzothiophene-pyrone type compound and synthesis method thereof
A technology of benzothiophene and pyrone, which is applied in the field of benzothienopyrone compounds and synthesis thereof, can solve the problems of narrow substrate range, low reaction yield, harsh reaction conditions and the like, achieves direct reaction, The effect of high yield and easy separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Preparation of 6-methyl-3-methyl-1H-benzo[4,5]thieno[3,2-c]pyran-1-one
[0041] Add 6-methyl-4-(4-methylphenylthio)pyrone (0.20mmol), palladium acetate (10mol%), copper trifluoromethanesulfonate (0.40mmol ) and ethanol (1ml) as solvent. Heat and stir at 140°C for 2 hours, monitor the progress of the reaction with TLC, extract with saturated sodium bicarbonate (3×5ml) and ethyl acetate (3×5ml) after the reaction is complete, combine the organic phases, dry and filter over anhydrous magnesium sulfate Concentrate and separate by silica gel column chromatography to obtain 37.71 mg of white powder with a yield of 82%. The structural formula of the product obtained is as follows:
[0042]
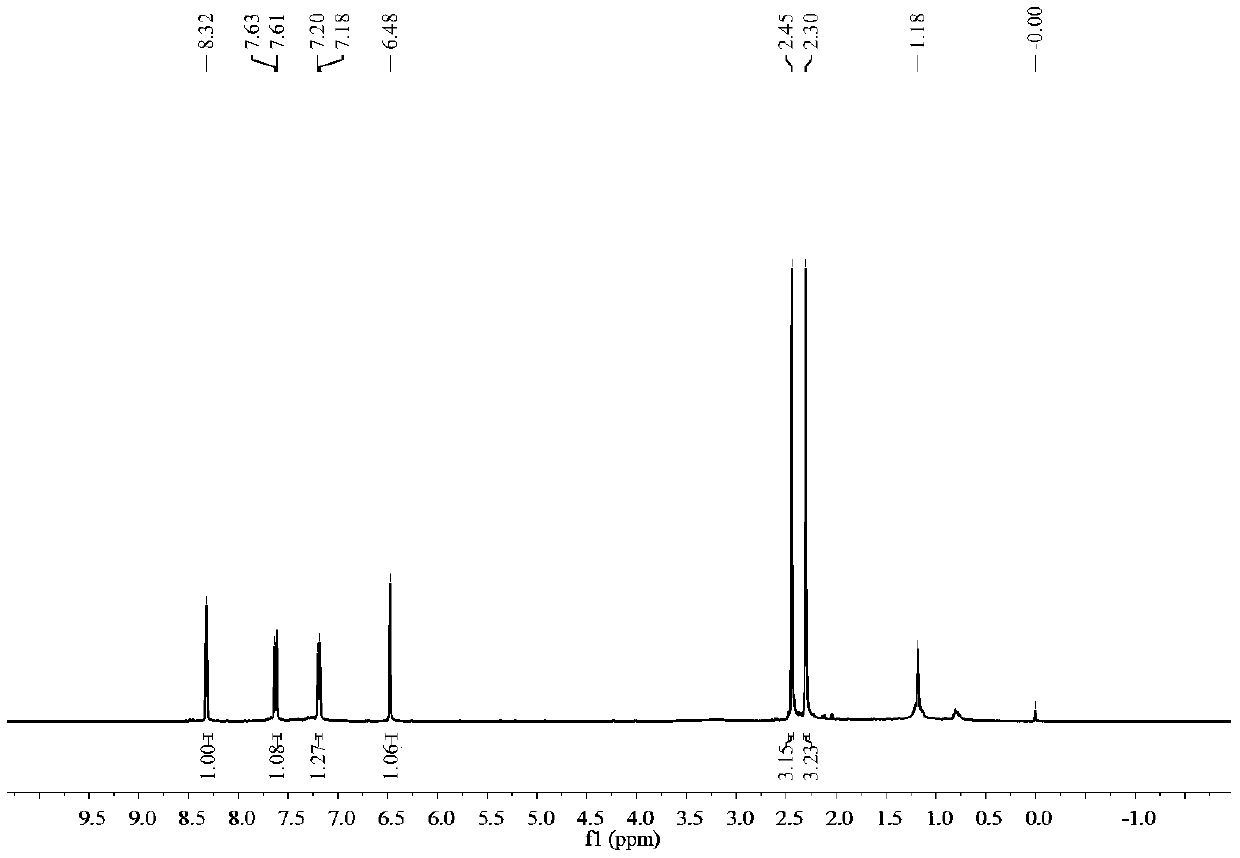
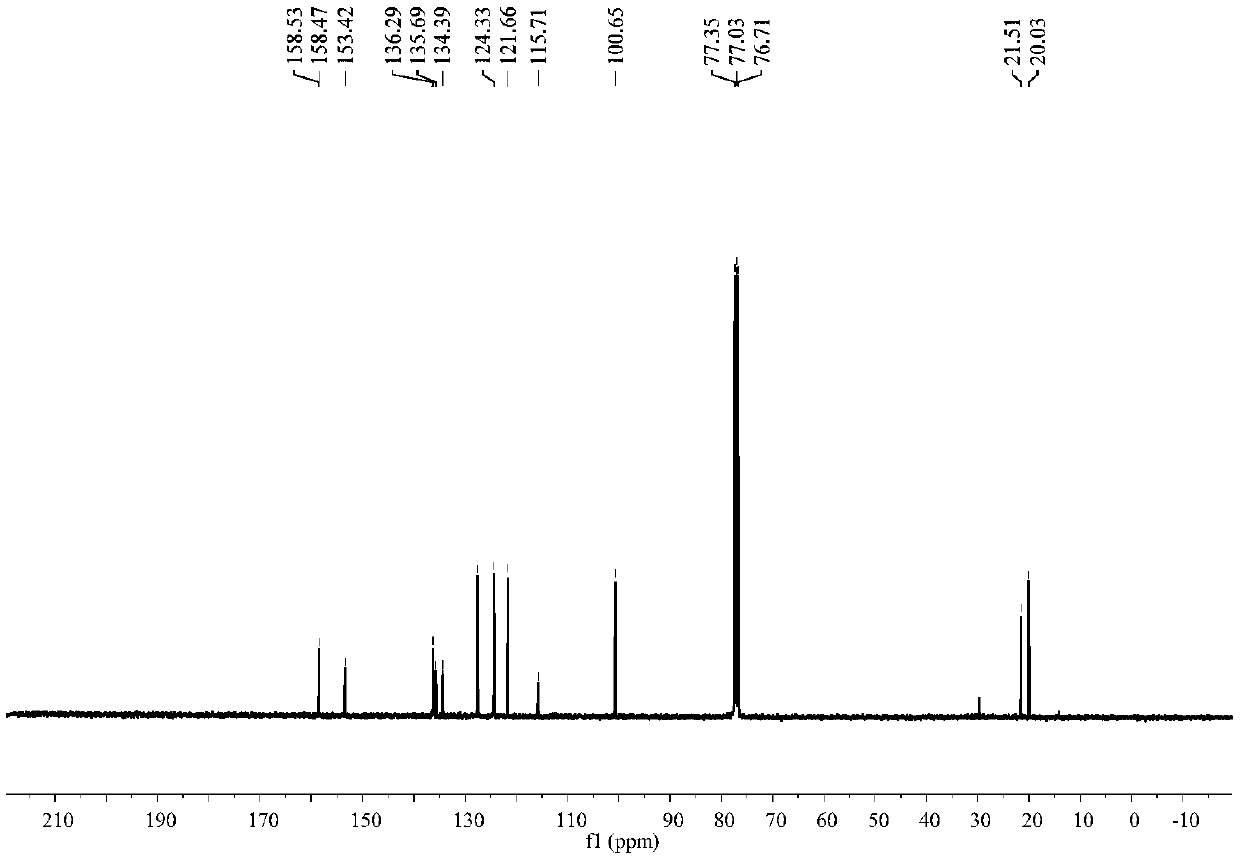
[0043] Such as figure 1 and figure 2 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3)δ8.32(s,1H),7.62(d,J=8.2Hz,1H),7.19(d,J=7.0Hz,1H),6.48(s,1H),2.45(s,3H),2.30( s,3H). 13 C NMR (101MHz, CDCl 3 )δ158.53, 158.47, 153.42, 136.29, 135.69, 134.39, 127.57, 124.33, 121....
Embodiment 2
[0045] Preparation of 8-bromo-3-methyl-1H-benzo[4,5]thieno[3,2-c]pyran-1-one
[0046] Add 6-methyl-4-(2-bromophenylthio)pyrone (0.20 mmol), palladium acetate (10 mol%), silver acetate (0.40 mmol) and dimethylmethylene to a 10 ml dry Schlenk tube, respectively. Sulfone (1ml) was used as solvent. Heat and stir at 140°C for 2 hours, monitor the progress of the reaction with TLC, extract with saturated sodium bicarbonate (3×5ml) and ethyl acetate (3×5ml) after the reaction is complete, combine the organic phases, dry and filter over anhydrous magnesium sulfate Concentrate and separate by silica gel column chromatography to obtain 38.21 mg of white powder with a yield of 65%. The structural formula of the resulting product is as follows:
[0047]
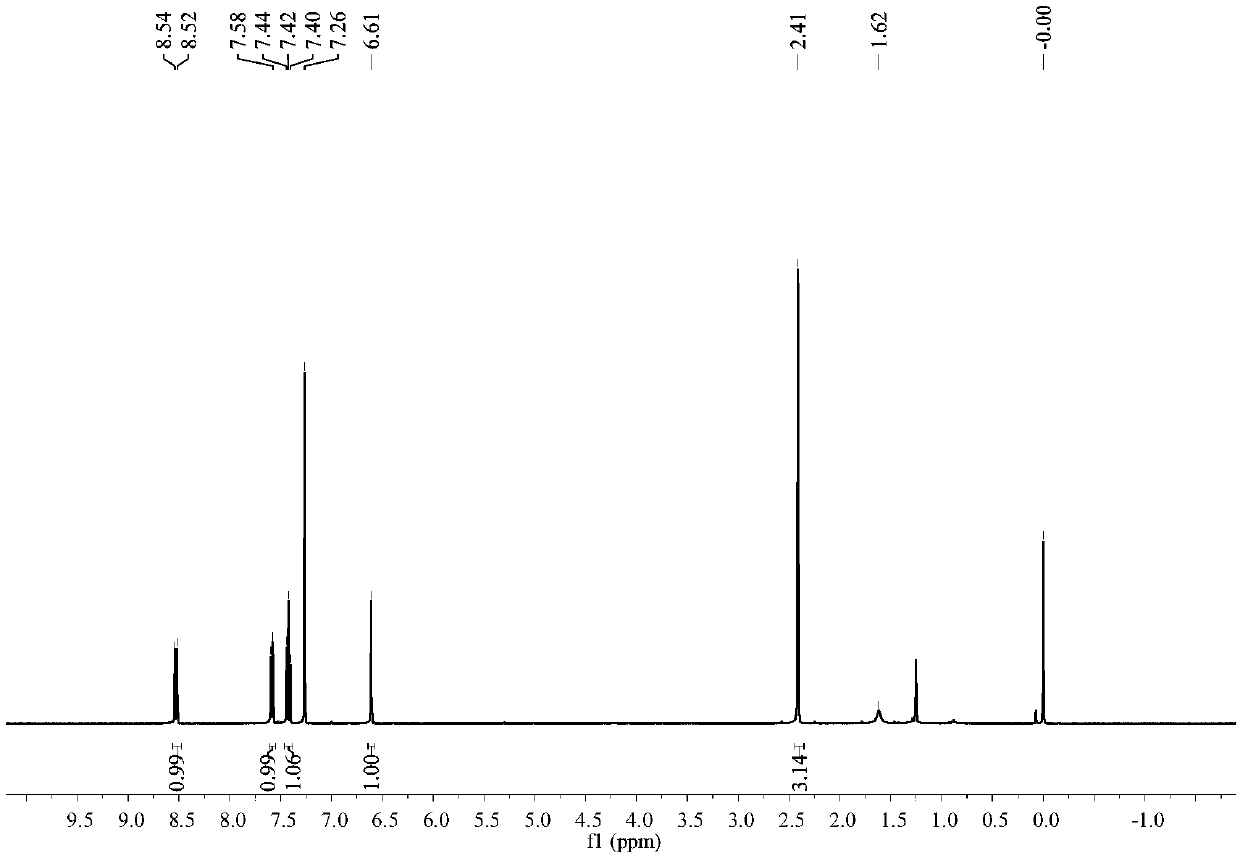
[0048] Such as image 3 and Figure 4 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ8.53(d, J=8.9Hz, 1H), 7.59(d, J=7.7Hz, 1H), 7.42(t, J=7.9Hz, 1H), 6.61(s, 1H), 2.41(s, 3H).13C NMR (101MHz, CDCl 3 )δ159.53, 1...
Embodiment 3
[0050] Preparation of 6-bromo-3-methyl-1H-benzo[4,5]thieno[3,2-c]pyran-1-one
[0051] Add 6-methyl-4-(4-bromophenylthio)pyrone (0.20mmol), palladium dichloride (15mol%), silver oxide (0.20mmol) and acetic acid ( 1.5ml) as solvent. Heat and stir at 110°C for 10 hours under sealing, monitor the progress of the reaction with TLC, extract with saturated sodium bicarbonate (3×5ml) and ethyl acetate (3×5ml) after the reaction is complete, combine the organic phases, dry and filter over anhydrous magnesium sulfate Concentrate and separate by silica gel column chromatography to obtain 42.91 mg of white powder with a yield of 73%. The structural formula of the resulting product is as follows:
[0052]
[0053] Such as Figure 5 and Figure 6 Shown, product NMR characterization: 1 H NMR (400MHz, CDCl 3 )δ8.36(d,J=8.0Hz,1H),7.51(td,J=8.0,5.1Hz,1H),7.22–7.12(m,1H),6.61(s,1H),2.41(s,3H ). 13 C NMR (101MHz, CDCl 3 )δ159.60, 158.48, 156.08, 153.69, 138.56, 127.96, 127.89, 123.97, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com