Engineered spider silk proteins and uses thereof
A spider silk protein and sequence technology, applied in biochemical equipment and methods, peptide sources, peptides, etc., can solve problems such as undetermined effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0254] Example 1: Generation of chimeric miniature spidroin NT2RepCT
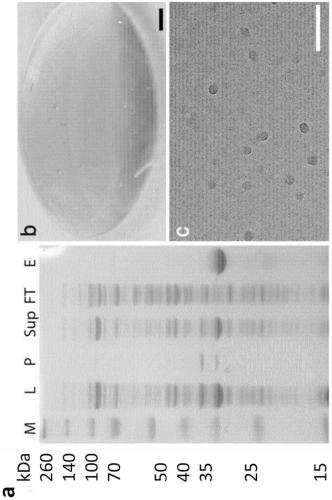
[0255] The present inventors designed a miniature spidroin protein comprising NT from E. australis MaSpl and CT from E. australis MiSp bracketing a short repeat region from E. australis. The chimeric NT2RepCT protein was produced at unprecedentedly high levels in shake flask E. coli cultures and the yield after purification was about 125 mg protein / L cell culture. Almost all proteins are soluble after expression and cleavage, and bind efficiently to Ni-NTA columns ( figure 2 A). The eluate contained >95% pure NT2RepCT, and the protein size on the SDS PAGE gel corresponded well to the expected molecular mass (33 kDa) ( figure 2 A). Size exclusion chromatography indicated a mass of 100 kDa ( Image 6 ), in good agreement with the dimer (due to the constitutive dimeric nature of CT) and the non-globular structure of the repeat.
Embodiment 2
[0256] Example 2: Chimeric miniature spidroin NT2RepCT exhibits extreme solubility
[0257] Obtaining high concentration of spinning dopes in water has been a long-term main goal, but to date, even though non-physiological solvents have been used, the concentration of artificial spinning dopes has been reported to be in the range of 10%-30%. In terms of solubility, the NT2RepCT of Example 1 far exceeds all expectations; it can be concentrated to 500 mg / ml in an aqueous buffer at pH 8 without precipitation out, a concentration equal to or even greater than that found in spider spinning dope protein concentration. At such high concentrations, the protein forms a yellow hydrogel ( figure 2 B). Natural spider silk stocks have been shown to store as micelles, 100nm-200nm in diameter, likely with terminal domains in the shell and repeat regions occluded in the core. This has also been proposed as a storage mechanism in silk glands. The NT2RepCT protein behaves like native silk ...
Embodiment 3
[0259] Example 3: Biomimetic spinning of NT2RepCT miniature spidroin
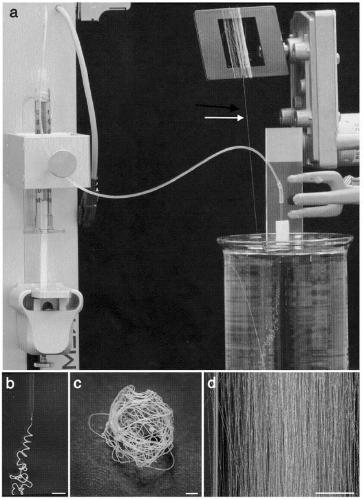
[0260] Another prerequisite that needs to be fulfilled in order to realize biomimetic spinning is to design a spinning device that can mimic the conditions of the spider silk gland. We designed a first-generation simple yet effective spinning device from a thin, drawn glass capillary through which a highly concentrated NT2RepCT stock solution was pumped into a collection bath of acidic aqueous buffer ( image 3 ). This setting causes a drop in pH and allows shear forces to act on the stock solution as it passes through the tip of the capillary and leads to the formation of continuous solid fibers ( image 3 A- image 3 B). Fibers can be easily wound in air onto a rotating frame in lengths exceeding hundreds of meters ( image 3 C). Fibers can be spun into dope concentrations ranging from 100 mg / ml to 500 mg / ml. Fibers spun from dopes with concentrations >200 mg / ml are easier to handle and can be spun ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| tenacity | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com