Preparation method of antibody coupled drug
An antibody-drug conjugated and conjugated technology, applied in the field of biomedicine, can solve the problem of high toxicity and achieve the effect of improving the growth inhibition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Preparation of anti-human ErbB2 bispecific antibody As3H
[0047] The sequence of the anti-human ErbB2 monoclonal antibody Trastuzumab (IgG1, κ) comes from the patent US5821337 of Genentech, and the anti-human ErbB2 monoclonal antibody HuA21 was created by Hefei Hankemaibo Biotechnology Co., Ltd. Its sequence comes from the patent ZL201410489895X.
[0048] As3H is a ScFv formed by connecting the variable regions of the light and heavy chains of HuA21 to the N-terminus of the heavy chain of Herceptin through a linker with a sequence of GGGGSGGGGSGGGGS. The amino acid sequence of the variable regions of the light and heavy chains in the ScFv is GGGGSGGGGSGGGGSGGGGS, and the anti-human ErbB2 double The schematic diagram of the structure of the specific antibody As3H is as follows figure 1 shown.
[0049] The construction process of the recombinant vector used to express the anti-human ErbB2 bispecific antibody As3H is as follows:
[0050] According to reports ...
Embodiment 2
[0054] Example 2. Preparation and condition optimization of antibody-conjugated drug As3H-MMAE
[0055] 1. Take a Sephadex G25FF column, equilibrate with 5 column volumes of pH7.4, 0.01M PBS buffer, then load the anti-human ErbB2 bispecific antibody As3H (ie, naked antibody), and then use Purified antibody solution was obtained by eluting with 1 column volume of 2 mM EDTA in pH 7.4, 0.01 M PBS buffer.
[0056] 2. After completing step 1, take the purified antibody solution and check the purity of the purified antibody by SDS-PAGE.
[0057] The results showed that the purified antibody solution was electrophoresis pure.
[0058] 3. After completing step 1, take the purified antibody solution and measure the concentration by BCA method.
[0059] 4. Mix an aqueous solution containing 10-50 mmol / L TCEP and 1 mM EDTA with 1 mL of purified antibody solution (concentration of 5 mg / mL) to obtain a mixed solution. In the mixture, the molar ratio of purified antibody and TCEP is 1:4,...
Embodiment 3
[0071]Embodiment 3, the scale-up production of antibody-conjugated drug As3H-MMAE
[0072] The scale-up production (about 200 mg) of antibody-conjugated drug As3H-MMAE is carried out according to the following production conditions:
[0073] Reduction conditions: the concentration of purified antibody is 5mg / mL, the molar ratio of purified antibody to TCEP is 1:5, room temperature;
[0074] Coupling conditions: the molar ratio of vc-MMAE and the reduced purified antibody is 7:1, the concentration of the reduced purified antibody is 2.5mg / mL, the reduction time is 60min, and the coupling time is 30min, the reduction step will be completed The mixture was diluted 2 times with pH7.4, 0.01M PBS buffer.
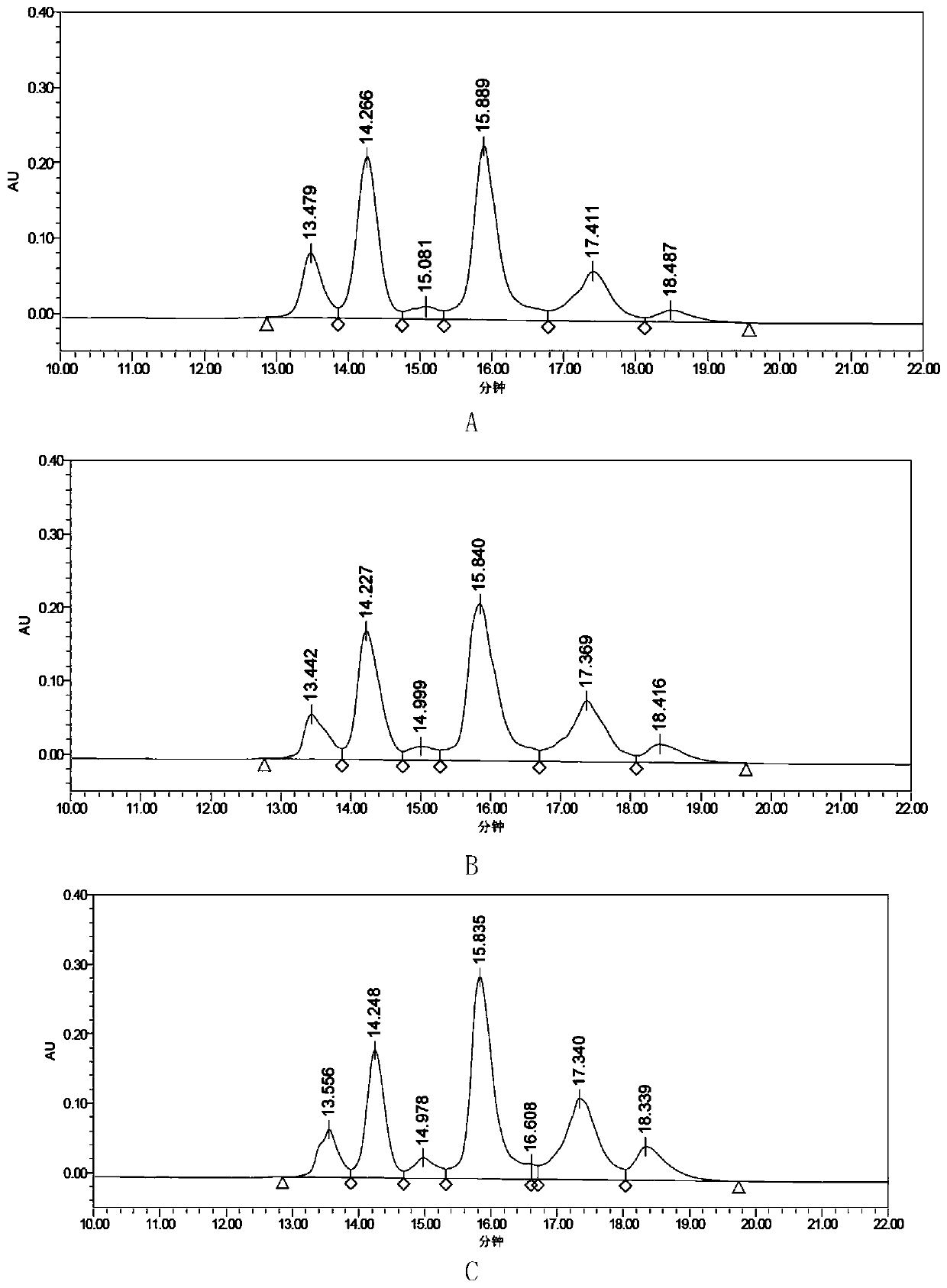
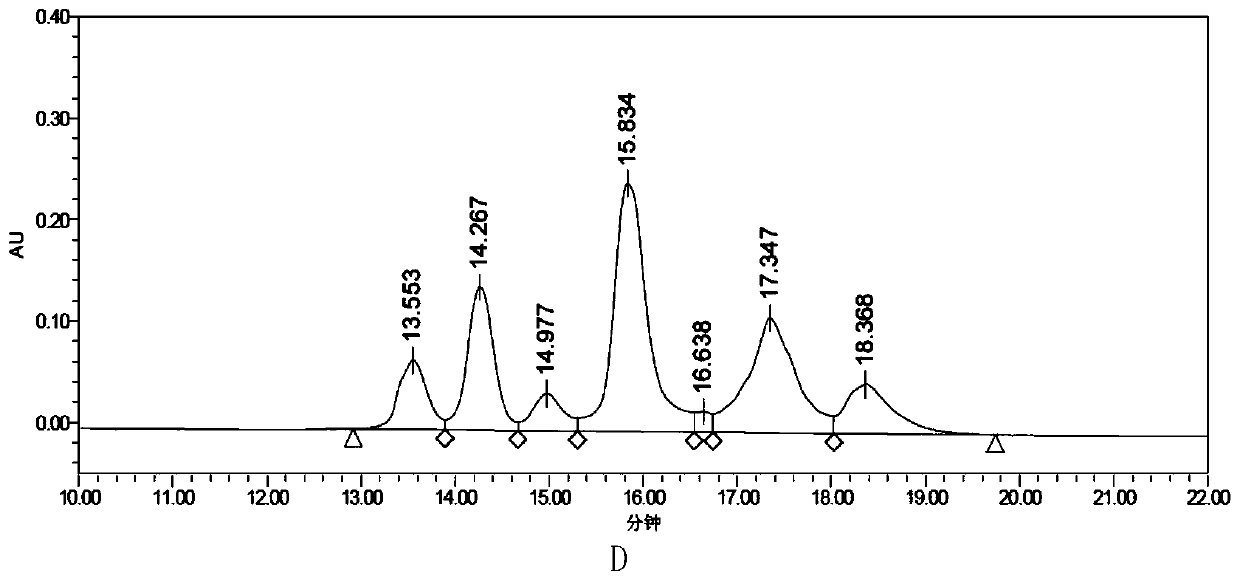
[0075] The purity of the antibody-conjugated drug As3H-MMAE and the average drug-conjugated number per antibody molecule were detected.
[0076] For the results of detecting the purity of the antibody-conjugated drug As3H-MMAE, see image 3 Middle A and Table 2. The results sh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com