A screening kit for primary open-angle glaucoma
A technology for open-angle glaucoma and a kit, applied in the field of SNP, can solve problems such as the association between glaucoma and RAMP2 gene mutation sites that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0032] Example Kit of the present invention and method of use
[0033] All components, contents and use methods in the kit of the present invention are as follows:
[0034] 1. PCR amplification reagents (50 copies):
[0035] The PCR amplification reagent is used to amplify a DNA sequence where the SNP site is located, and its composition is shown in Table 1.
[0036] Table 1 PCR amplification reagents
[0037] The PCR mixed solution in Table 1 includes the required components of conventional PCR such as Taq enzyme, dNTP, magnesium ion;
[0038]
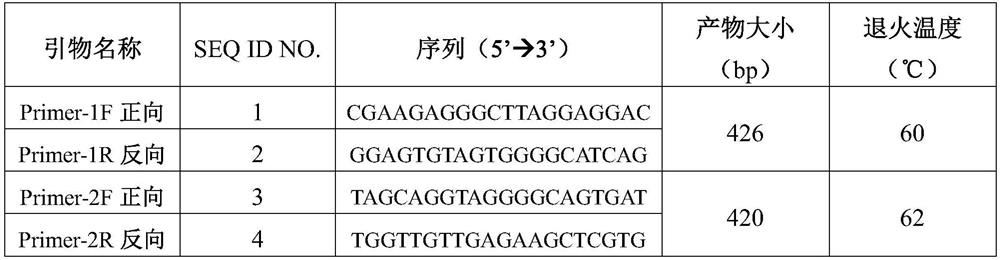
[0039] The primer pair information is shown in Table 2.
[0040] Table 2 Primers used for RAMP2 gene amplification
[0041]
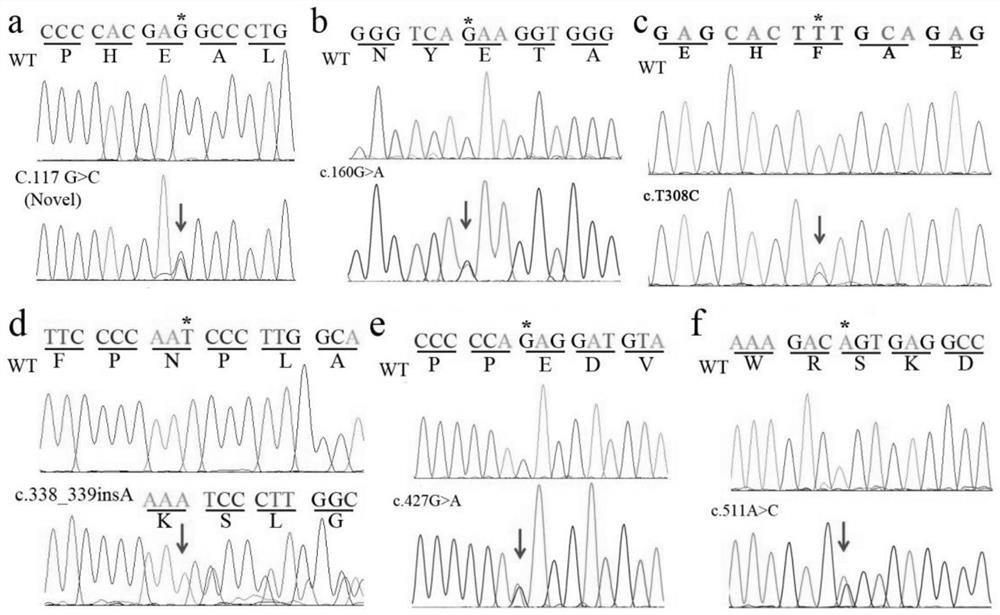
[0042] Attachment: Primer-1F / R primer amplification: Glu39Asp (c.117G>C), Glu54Lys (c.160G>A)
[0043]Primer-2F / R primer amplification: Phe103Ser (c.308T>C), Asn113Lysfs*10 (c.338_339insA), Glu143Lys (c.427G>A), Ser171Arg (c.511A>C)
[0044] 2. RAMP2 gene variant typing detection reagent (50 copies)...
experiment example 1
[0077] Experimental Example 1 Screening of mutation sites
[0078] 1. Method
[0079] DNA was extracted from 4763 POAG patients and 10953 healthy controls. Through next-generation sequencing technology, Illumina TruSeq is used to enrich and capture, and the HiSeq 2000 / 2500 sequence generator is used to target and screen POAG candidate genes, so that the genomic DNA of each individual is enriched in the library. Libraries were sequenced using an automated Illumina HiSeq 2000 / 2500 sequencer. The sequencing sequence results were consistent with the UCSC hg19 (http: / / genome.ucsc.edu / ) sequence using BWA-MEM (https: / / github.com / lh3 / bwa). SNPs and Indels were detected by using two databases, Samtools (http: / / samtools.sourceforge.net / ) and freebayes (https: / / github.com / ekg / freebayes). Based on the information in the RefSeq database, we used the Annovar (http: / / annovar.openbioinformatics.org / en / latest / ) database to classify SNVs into different functional categories based on their g...
experiment example 2
[0084] Experimental Example 2 The specificity test of the kit of the present invention for the detection of RAMP2 genotyping
[0085] 1. Method
[0086] The mutant sample DNA involved in Table 2 in Experimental Example 1 and the DNA of 20 healthy subjects were used as experimental materials, and the kits in the Examples were used for detection according to the above-mentioned experimental methods.
[0087] 1) DNA extraction
[0088] Take 2ml of patient's whole blood (EDTA anticoagulation), and extract its genomic DNA.
[0089] 2) Amplify the DNA fragment containing the detected SNP site by PCR, and the PCR amplification system of each mutation site is as follows:
[0090]
[0091]
[0092] Reaction conditions:
[0093]
[0094] PCR product detection:
[0095] The PCR product was detected by 2% agarose gel electrophoresis, the effect of the PCR reaction was observed, and the amount added as a template in the subsequent reaction was determined.
[0096] 3) Sanpshot...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com