Human agrin antigen, human agrin antibody detection kit, preparation method and application thereof
A technology of antigen and carrier, applied in the field of biopharmaceuticals, can solve the problem of not being detected, and achieve the effect of strong specificity, good stability and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1 Construction of recombinant expression plasmid and engineering bacteria
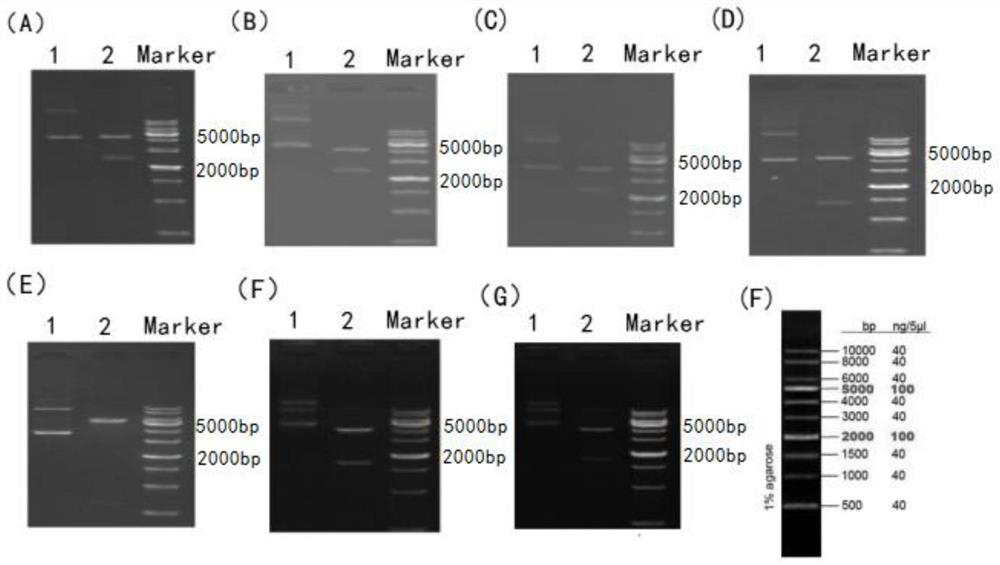
[0055] 1. Agrin is a heparan sulfate proteoglycan composed of multiple domains, including 9 protein kinase inhibitor domains, 4 epidermal growth factor-like domains and 1 adhesion molecule G homology domain. The mature Agrin protein is composed of 2038 amino acids. In this study, the amino acid sequence of Agrin protein was analyzed including hydrophilicity and surface accessibility, and combined with its spatial conformation and the modification characteristics of each domain, fragments of 7 regions of mature Agrin protein were selected to obtain them respectively. After a series of studies, the inventors obtained seven human Agrin antigens, Agrin-40, Agrin-411, Agrin-701, Agrin-1103, Agrin-1281, Agrin-1635 and Agrin-1864.
[0056] 2. PCR amplification of human Agrin antigen gene
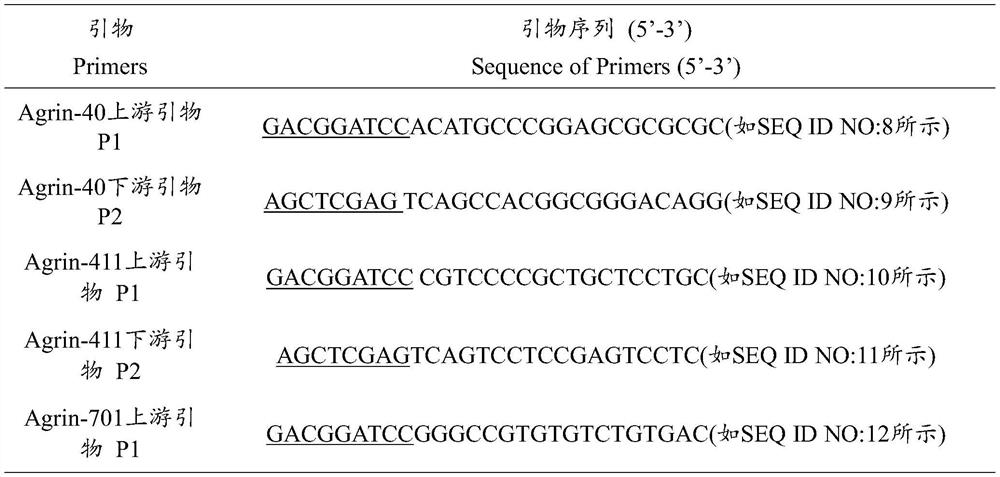
[0057] 1. The DNA sequences of the 7 fragments were respectively subjected to gene synthesis, and the p...
Embodiment 2
[0066] Example 2 Expression and purification of antigen
[0067] 1. Use the constructed recombinant protein expression engineering bacteria to conduct the induction expression experiment. Inoculate 7 recombinant bacteria in 600ml of LB broth (components: 10g sodium chloride / liter, 10g peptone / liter and 5g yeast extract / liter), shake the bacteria at 37 degrees and 200RPM to OD600 to 0.6-0.8, press IPTG at a concentration of 24 mg / ml was added at 1:1000 for induction for 4 hours. Centrifuge, harvest bacteria, and prepare for purification.
[0068] 2. The selected filler for purification is GE's Ni Sepharose (Item No. 17-0729-10), and the following solutions are prepared according to its instructions:
[0069] Loading Buffer A: 0.5M NaCl + 20mM Na 2 HPO 3 +10 mM imidazole.
[0070] Binding buffer B: 0.5M NaCl + 20mM Na 2 HPO 3 +20mM imidazole
[0071] Elution buffer C: 0.5M NaCl, 20mM Na 2 HPO 3 , 500mM imidazole;
[0072] and the solution used to purify inclusion bodi...
Embodiment 3
[0078] Embodiment 3 Human Agrin antibody detection kit and method of use
[0079] 1. Coated ELISA plate
[0080] (1) Coating solution: NaCl 8.5g, Na 2 HPO 4 ·12H 2 O 30.8g, KH 2 PO 4 2.2g, add ddH 2 O to 1000ml, adjust pH to 7.4.
[0081] (2) Lotion for coating: NaCl 8.0g, KH 2 PO 4 0.24g, Na 2 HPO 4 ·12H 2 O 2.9g, KCl 0.2g, TWEEN200.5ml, add to ddH 2 O to 1000ml, adjust to PH7.4.
[0082] (3) Coating method: 7 Agrin antigen fragments were respectively coated in the microplate wells with 0.1M PBS (PH7.4) coating buffer, overnight at 4 degrees, among which Agrin-25, Agrin-344, The coating concentrations of Agrin-530, Agrin-824 and Agrin-1048 were 200ng / ml, 200ng / ml, 250ng / ml, 150ng / ml and 250ng / ml, respectively. Add 100 μL to each well of a 96-well microtiter plate, and set it to adsorb at 2-8°C for 24 hours. Empty the coating solution and wash the plate 3 times with washing solution.
[0083] 2, closed
[0084] (1) Blocking solution for coating: Na 2 HPO 4 ·...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com