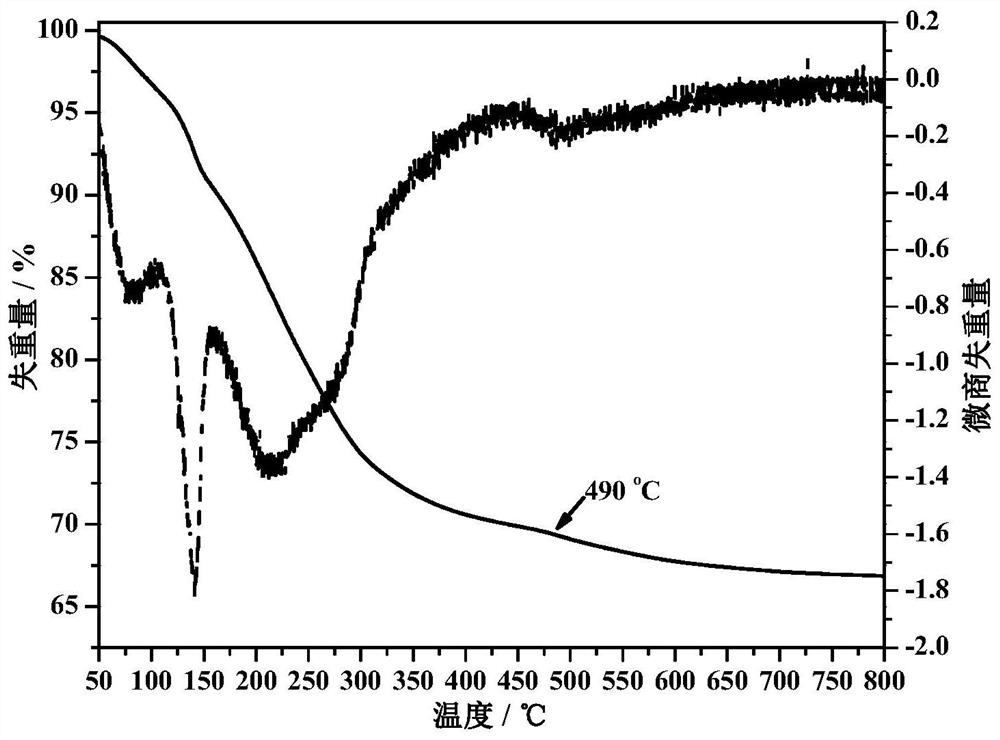
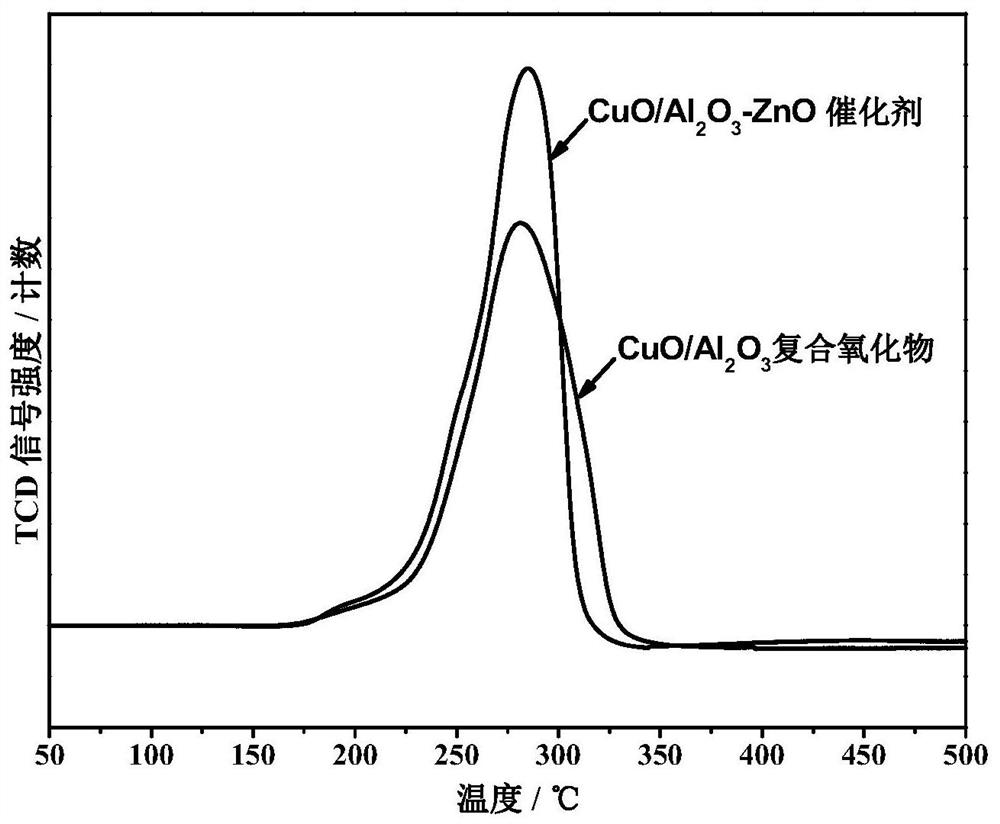
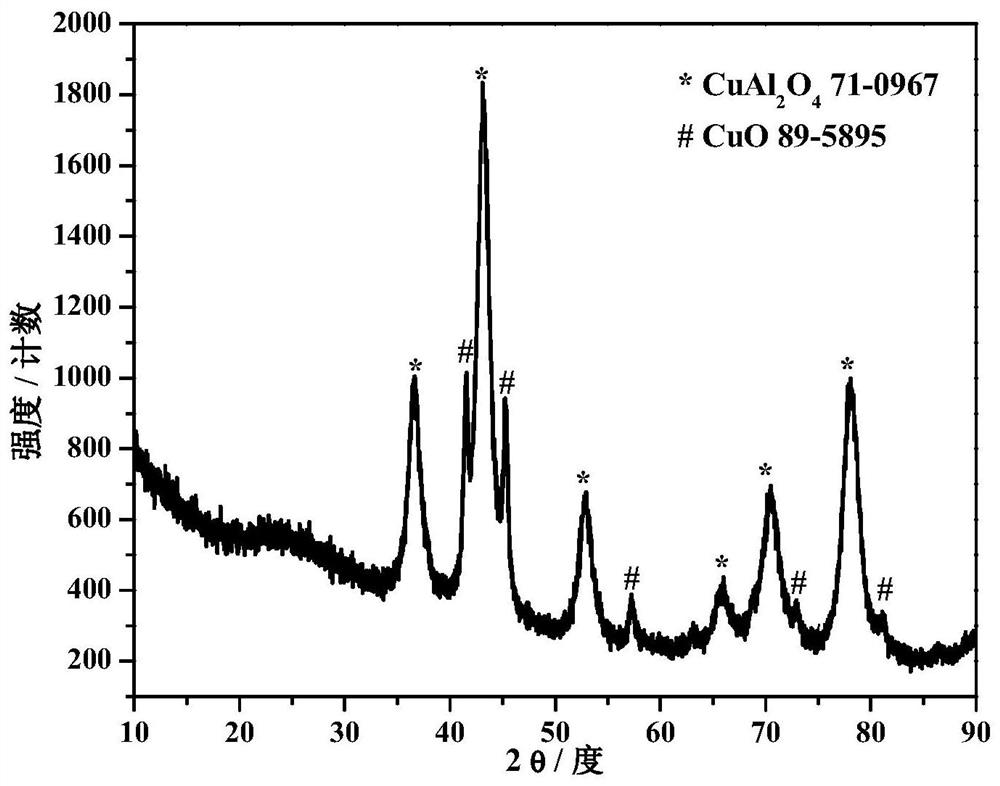
Preparation method of cu-based water gas shift reaction catalyst
A transformation reaction, water gas technology, applied in catalyst activation/preparation, chemical instruments and methods, physical/chemical process catalysts, etc., can solve the problems of poor performance reproducibility, environmental pollution, etc., and achieve stable structure and performance, high activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Accurately weigh the mass to be 18.2248g Cu(NO 3 ) 2 ·3H 2 O and 66.2234g Al(NO 3 ) 3 9H 2 O was prepared into 400ml mixed brine solution and 55.0138g Na 2 CO 3 Add dropwise to 500ml of deionized water; use the co-current co-precipitation method, control the temperature of the water bath at 80°C, and mechanically stir, and the pH value of the reaction system is 7.5. After the above solution was added dropwise, the reaction was continued for 3 h under stirring conditions. Naturally cooled, the precipitate was centrifuged, washed, dried at 110°C for 10h, and calcined in a static air atmosphere at 500°C for 6h to obtain CuO-CuAl-containing 2 o 4 / Al 2 o 3 of composite oxides.
[0033] Weigh 8g containing CuO-CuAl 2 o 4 / Al 2 o 3 composite oxide, add 25ml Zn(NO 3 ) 2 solution (0.87mol / L), impregnated and adsorbed at room temperature for 3h, dried at 110°C for 10h, and roasted in a static air atmosphere at 400°C for 3h to obtain CuO-CuAl-containing 2 o 4 / ...
Embodiment 2
[0037] Accurately weigh the mass to be 17.1960g Cu(NO 3 ) 2 ·3H 2 O and 68.7399g Al(NO 3 ) 3 9H 2 O was prepared by dissolving 400ml of mixed salt solution and 29.1273g of KOH in 500ml of deionized water; using the co-current co-precipitation method, controlling the temperature of the water bath at 80°C, stirring mechanically, and the pH of the reaction system was 8.0. After the salt solution was added dropwise, the reaction was continued for 2 h under stirring conditions. Naturally cooled, the precipitate was centrifuged, washed, dried at 110°C for 10h, and calcined in a static air atmosphere at 800°C for 2h to obtain CuO-CuAl-containing 2 o 4 / Al 2 o 3 of composite oxides.
[0038] Weigh 8g containing CuO-CuAl 2 o 4 / Al 2 o 3 composite oxide, add 20ml Zn(OAc) 2 solution (1.23mol / L), impregnated and adsorbed at room temperature for 3h, dried at 110°C for 10h, and roasted in a static air atmosphere at 400°C for 2h to obtain CuO-CuAl-containing 2 o 4 / Al 2 o 3...
Embodiment 3
[0041] Accurately weigh 12.0856g Cu(NO 3 ) 2 ·3H 2 O and 37.5152g Al(NO 3 ) 3 9H 2 O prepared into 400ml mixed salt solution (Cu 2+ :Al 3+ =1:2) and 31.7802g Na 2 CO 3 Dissolve in 500ml of deionized water; use the co-current co-precipitation method, control the temperature of the water bath at 80°C, and mechanically stir, the pH of the reaction system is 7.0. After the salt solution was added dropwise, the reaction was continued for 4 h under stirring conditions. Naturally cooled, the precipitate was centrifuged, washed, dried at 110°C for 10h, and roasted in a static air atmosphere at 650°C for 2h to obtain CuO-CuAl-containing 2 o 4 / Al 2 o 3 of composite oxides.
[0042] Weigh 8g containing CuO-CuAl 2 o 4 / Al 2 o 3 composite oxide, add 25ml Zn(OAc) 2 solution (1.31mol / L), impregnated and adsorbed at room temperature for 3h, dried at 110°C for 10h, and roasted in a static air atmosphere at 450°C for 3h to obtain CuO-CuAl-containing 2 o 4 / Al 2 o 3 -ZnO t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com