Liquid crystal polyester, liquid crystal polyester composition and products prepared from liquid crystal polyester composition
A technology of liquid crystal polyester and repeating units, applied in the liquid crystal polyester composition and its products, in the field of liquid crystal polyester, can solve the problems of increasing cost, affecting the composition, easy foaming of liquid crystal polyester, etc. Anti-foaming performance, reduced small molecule content, effect of excellent anti-foaming performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
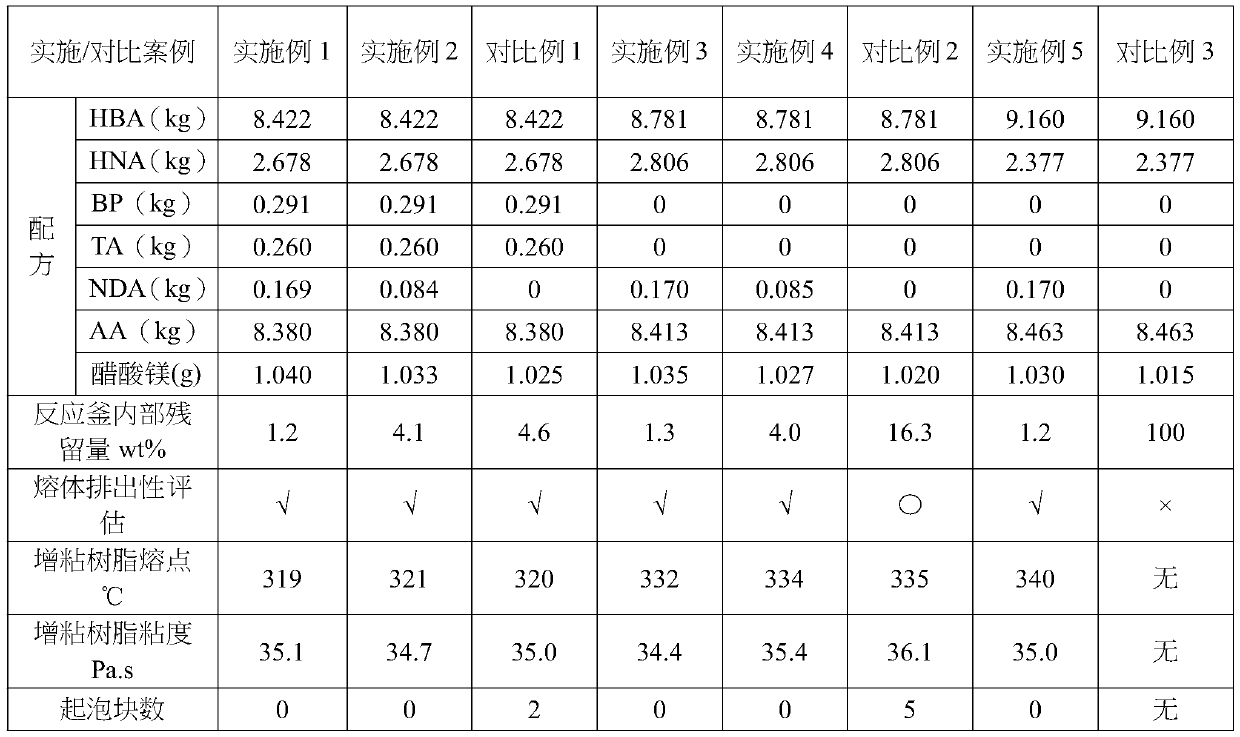
[0059] Add monomer, acylating agent and catalyst to reaction kettle one equipped with stirrer, thermometer, monomer feeding port, nitrogen inlet pipe and reflux condensing device respectively. The individual weights are as follows:
[0060] 4-hydroxybenzoic acid (HBA) 8.422kg,
[0061] 2-hydroxy-6-naphthoic acid (HNA) 2.678kg,
[0062] 4,4'-Dihydroxybiphenyl (BP) 0.291kg,
[0063] 2,6-naphthalene dicarboxylic acid (NDA) 0.169kg,
[0064] Acetic anhydride (AA) 8.380kg,
[0065] Magnesium acetate 1.040g,
[0066] After feeding, put nitrogen into the reaction kettle for replacement; start stirring, and heat the reaction mixture from room temperature to 140°C in nitrogen flow, and reflux at this temperature for 2 hours; after the acetylation reaction, transfer the material to a The stirrer of the torque sensor, the nitrogen inlet, the extraction device and the vacuum device reactor two. 0.260 kg of terephthalic acid (TA) has been put into reactor two in advance, and then the...
Embodiment 2
[0069] Add monomer, acylating agent and catalyst to reaction kettle one equipped with stirrer, thermometer, monomer feeding port, nitrogen inlet pipe and reflux condensing device respectively. The individual weights are as follows:
[0070] 4-hydroxybenzoic acid (HBA) 8.422kg,
[0071] 2-hydroxy-6-naphthoic acid (HNA) 2.678kg,
[0072] 4,4'-Dihydroxybiphenyl (BP) 0.291kg,
[0073] 2,6-naphthalene dicarboxylic acid (NDA) 0.084kg,
[0074] Acetic anhydride (AA) 8.380kg,
[0075] Magnesium acetate 1.033g,
[0076] After feeding, put nitrogen into the reaction kettle for replacement; start stirring, and heat the reaction mixture from room temperature to 140°C in nitrogen flow, and reflux at this temperature for 2 hours; after the acetylation reaction, transfer the material to a The stirrer of the torque sensor, the nitrogen inlet, the extraction device and the vacuum device reactor two. 0.260 kg of terephthalic acid (TA) has been put into reactor two in advance, and then the...
Embodiment 3
[0079] Add monomer, acylating agent and catalyst to reaction kettle one equipped with stirrer, thermometer, monomer feeding port, nitrogen inlet pipe and reflux condensing device respectively. The individual weights are as follows:
[0080] 4-hydroxybenzoic acid (HBA) 8.781kg,
[0081] 2-hydroxy-6-naphthoic acid (HNA) 2.806kg,
[0082] 2,6-naphthalene dicarboxylic acid (NDA) 0.170kg,
[0083] Acetic anhydride (AA) 8.413kg,
[0084] Magnesium acetate 1.035g,
[0085] After feeding, put nitrogen into the reaction kettle for replacement; start stirring, and heat the reaction mixture from room temperature to 140°C in nitrogen flow, and reflux at this temperature for 2 hours; after the acetylation reaction, transfer the material to a The stirrer of the torque sensor, the nitrogen inlet, the extraction device and the vacuum device reactor two. The mixture was heated from 145°C to 360°C over 3.5-5.0 hours during which time the metered acetic acid recovery = 95.7%. In the polyco...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com