1,3-diaryl pyrazole PTP1B inhibitor containing carboxyalkyl rhodanine structure as well as preparation and application thereof
A technology of pyrazole and structural formula, applied in the field of 1,3-diarylpyrazole PTP1B inhibitors containing carboxyalkyl rhodanine structure and its preparation and application, can solve the adverse effect and limit the value of treatment Drugs, poor selectivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
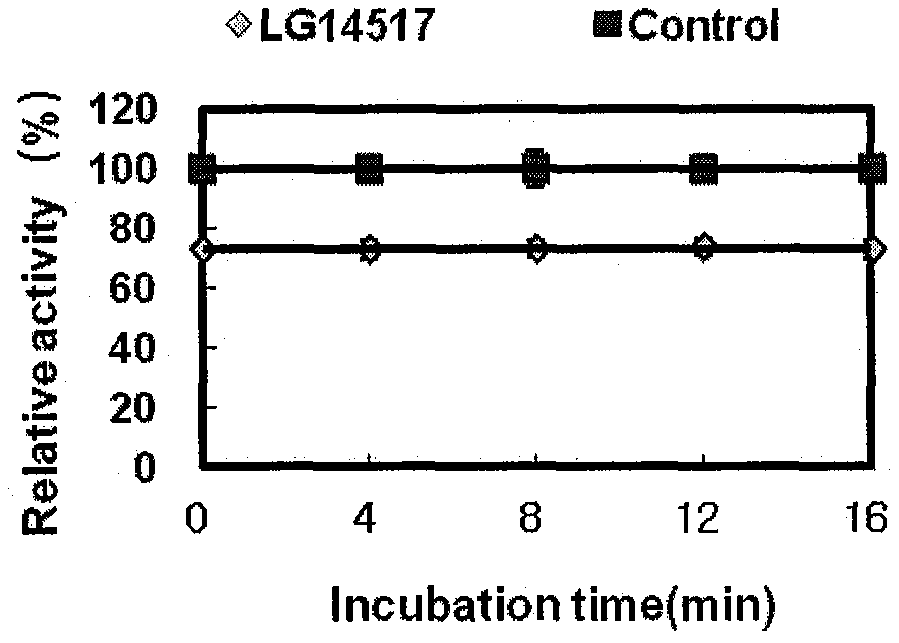
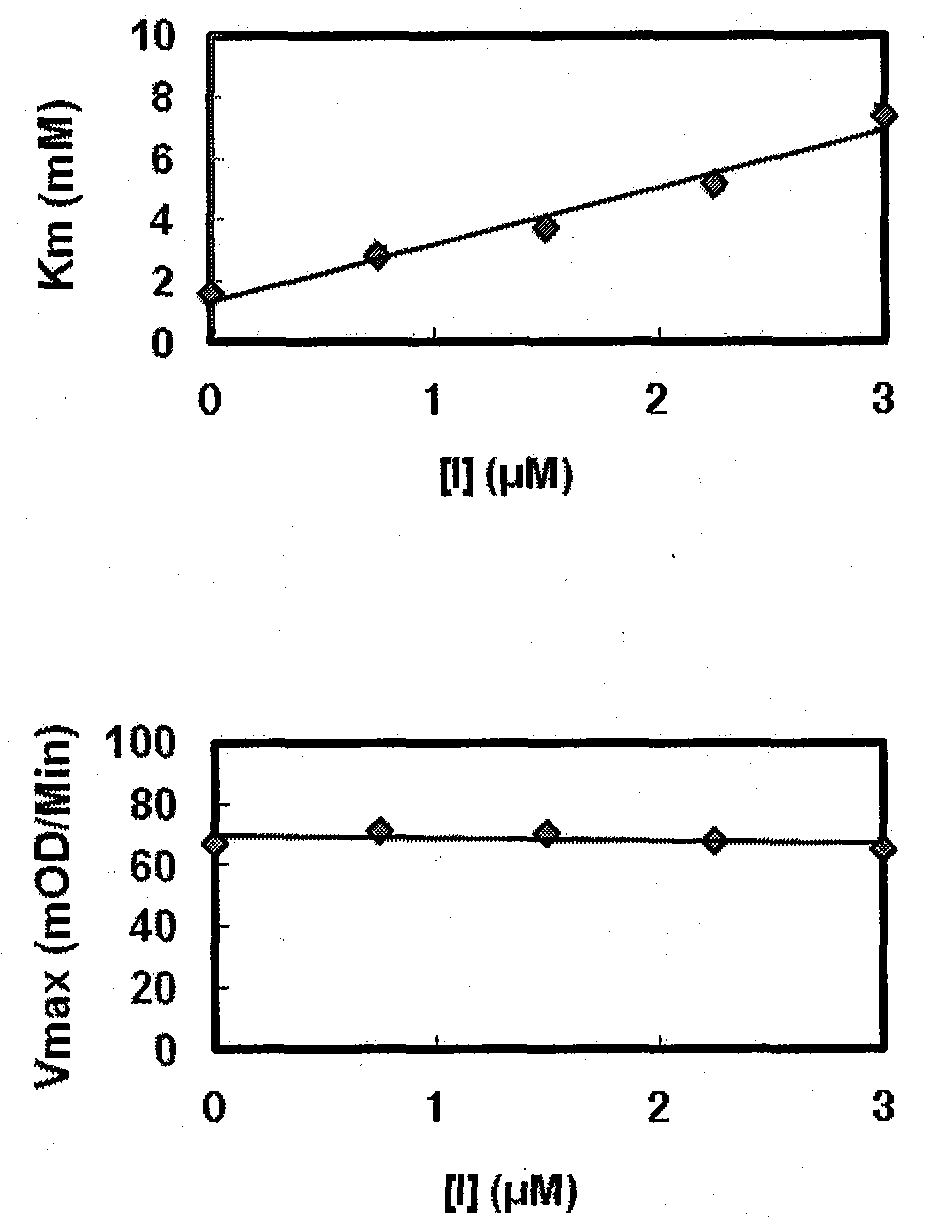
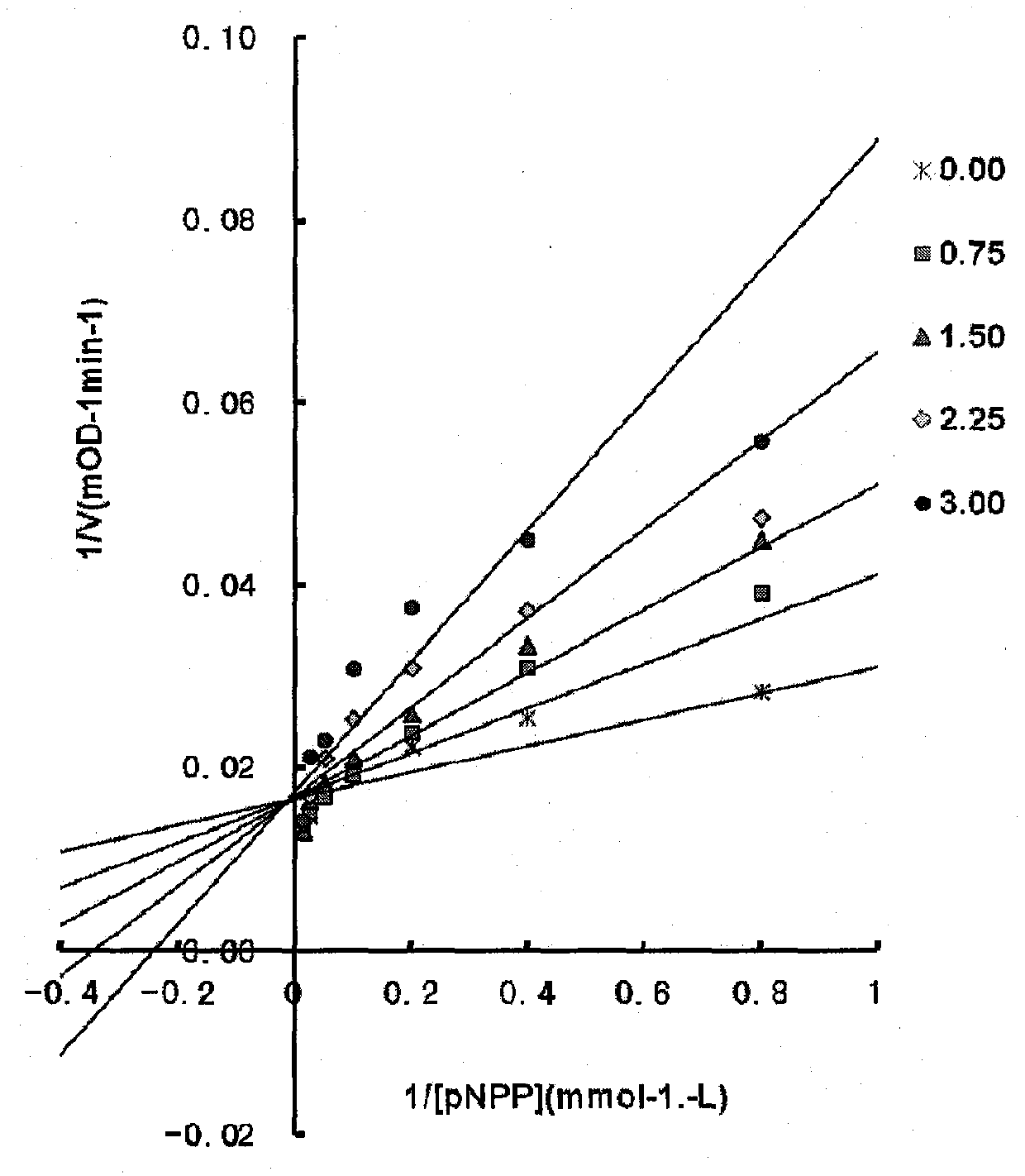
Image
Examples
Embodiment 1
[0022] Example 1: (Z)-2-(5-((3-(naphthalene-2-yl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-thio-4 -Thiazolidinone-3-yl)acetic acid (6a) Dissolve glycine (10mmol, 0.75g) in 15mL water, add NaOH (0.8g, 20mmol), slowly add 0.6mL CS 2 (10 mmol), stirred at room temperature for 10 hours. Anhydrous sodium chloroacetate (0.82 g, 10 mmol) was added in batches to the reaction solution, and stirring was continued at room temperature for 3 hours. Add 6M HCl (30mL), N 2 Protection, stirring and reflux for 16 hours, cooling. The reaction solution was extracted with ethyl acetate (3*50mL), the organic phases were combined, dried over anhydrous magnesium sulfate, filtered, and the filtrate was spin-dried to obtain a yellow-green oil. The crude product was subjected to silica gel column chromatography (eluent: dichloromethane: Methanol=20:1) to obtain 1.24 g of white solid (2a), with a yield of 65%.
[0023] Phenylhydrazine (0.57g, 5.3mmol) was dissolved in 20mL of absolute ethanol, 2'-napht...
Embodiment 2
[0026] Example 2: (Z)-2-(5-((3-(3-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-thio-4 -Thiazolidinone-3-yl) acetic acid (6b) replaces 2'-naphthylophenone with 3-chloroacetophenone, and the others are the same as 6a to obtain compound 6b with a yield of 91%; m.p.313-315°C. IR (KBr)cm -1 : 3419(OH), 1707(C=O). 1 H NMR (DMSO-d 6 , 300MHz, ppm): δ8.84(s, 1H), 8.06 (d, J=8.1Hz, 2H), 7.74(s, 1H), 7.61-7.53(m, 6H), 7.43(t, J=7.35 Hz, 1H), 4.49(s, 2H). 13 C NMR (DMSO-d 6 , 75MHz, ppm): δ245.30, 192.60, 167.12, 166.25, 152.27, 138.53, 133.68, 133.15, 130.98, 129.80, 122.63, 119.47, 115.74, 109.53, 40.20, 38.94.MS6 m / z+ .Calcd. for C 21 h 14 ClN 3 o 3 S 2 : C, 55.32; H, 3.09; N, 9.22; S, 14.07.Found: C, 55.31; H, 3.07; N, 9.20; S, 14.08.
Embodiment 3
[0027] Example 3: (Z)-2-(5-((3-(3-bromophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-2-thio-4 -Thiazolidinone-3-yl)acetic acid (6c) replaced 2′-naphthophenone with 3-bromoacetophenone, and the others were the same as 6a to obtain compound 6c with a yield of 88%; m.p.315-317°C. IR (KBr)cm -1 : 3419(OH), 1707(C=O). 1 H NMR (DMSO-d 6 , 300MHz, ppm): δ8.83(s, 1H), 8.07 (d, J=7.6Hz, 2H), 7.87(d, J=1.6Hz, 1H), 7.74(m, 1H), 7.66(d, J=7.9Hz, 1H), 7.54(m, 4H), 7.43(t, J=7.4Hz, 1H), 4.35(s, 2H).MS m / z 501(M+1).Calcd.for C 21 h 14 BrN 3 o 3 S 2 : C, 50.41; H, 2.82; N, 8.40; S, 12.82.Found: C, 50.39; H, 2.80; N, 8.38; S, 12.81.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com