High-purity poly IC lyophilized preparation and preparation method thereof
A technology for freeze-dried preparations and polymyocytes, which is applied in the field of high-purity polymyocytes freeze-dried preparations and their preparation, can solve the problem of heating of PIC products, and achieve the effects of reducing impurities, simplifying the production process and prolonging the storage time.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
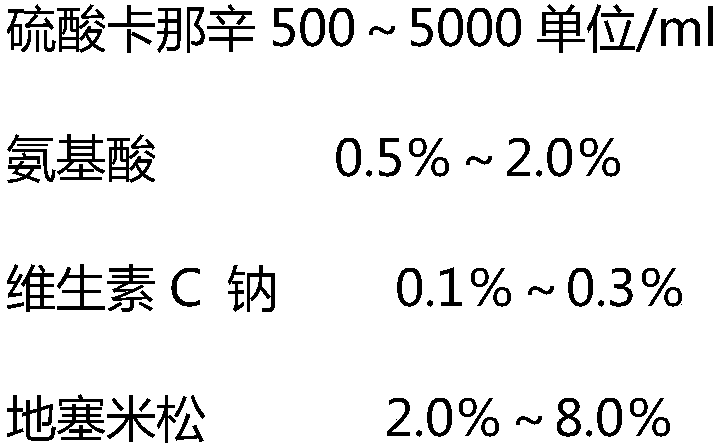
[0025] Take the purified solution of polyinosinic acid and polycytidylic acid (1:0.5), mix them in a water bath at 40°C, stir for 45 minutes, filter with a 0.45 μm filter element, and concentrate with a 300KD membrane bag of the Millipore ultrafiltration system to a concentration of 0.8mg / ml of liquid, add cannacin sulfate 500 units / ml, amino acid 0.5%, sodium ascorbate 0.15%, dexamethasone 2.0%, mix well, sterile filter, subpackage and freeze-dry.
Embodiment 2
[0027] Take the purified solution of polyinosinic acid and polycytidylic acid (1:1), mix them in a water bath at 50°C, stir for 60 min, filter with a 0.45 μm filter element, and concentrate to a concentration of 2.0 mg / ml of liquid, add cannacin sulfate 3000 units / ml, amino acid 1.0, sodium ascorbate 0.2%, dexamethasone 3.8%, mix well, sterile filter, subpackage and freeze-dry
Embodiment 3
[0029] Take the purified solution of polyinosinic acid and polycytidylic acid (1:1.5), mix them in a water bath at 50°C, stir for 90 min, filter with a 0.45 μm filter element, and concentrate to a concentration of 4.0 mg / ml of liquid, add cannacin sulfate 5000 units / ml, amino acid 1.0%, sodium ascorbate 0.3%, dexamethasone 6.0%, mix well, sterile filter, aliquot and freeze-dry
[0030] The finished freeze-dried preparation is tested according to the method listed in the "Standard" and is qualified.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com