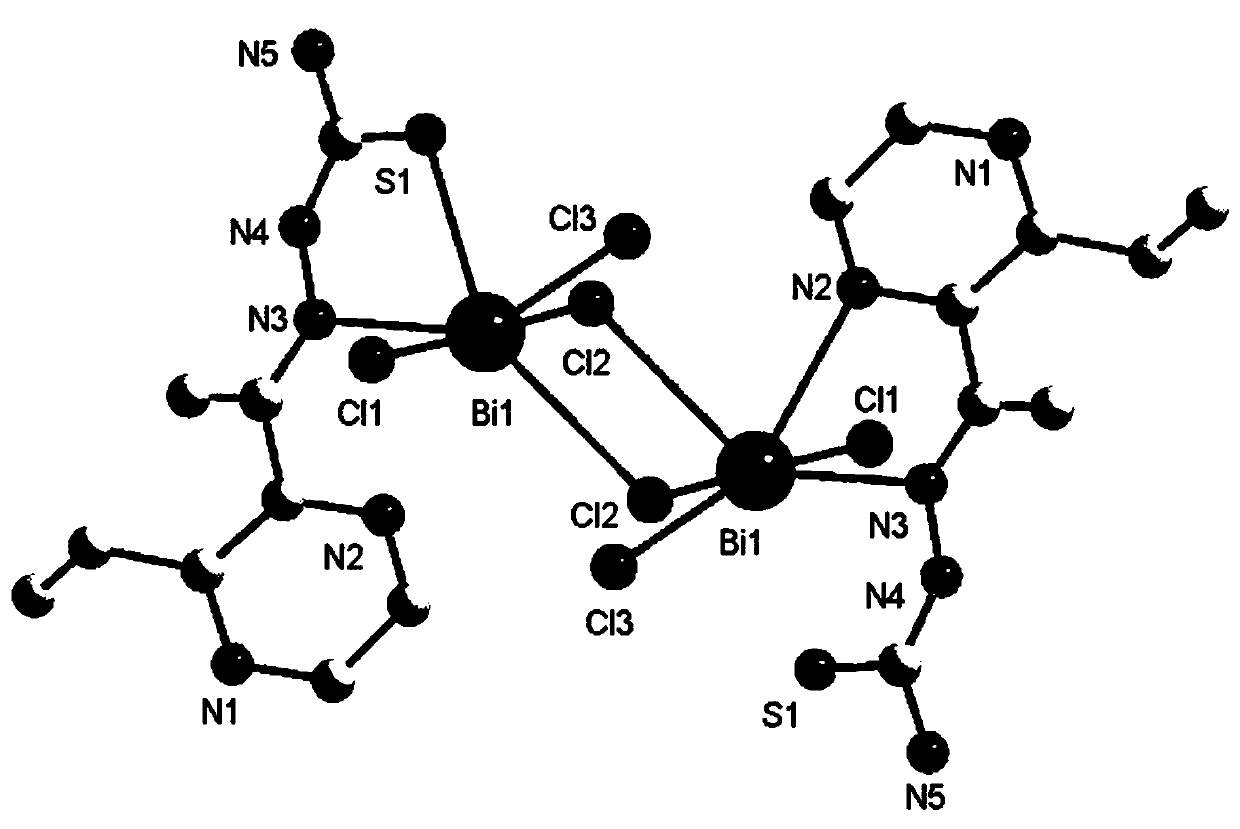
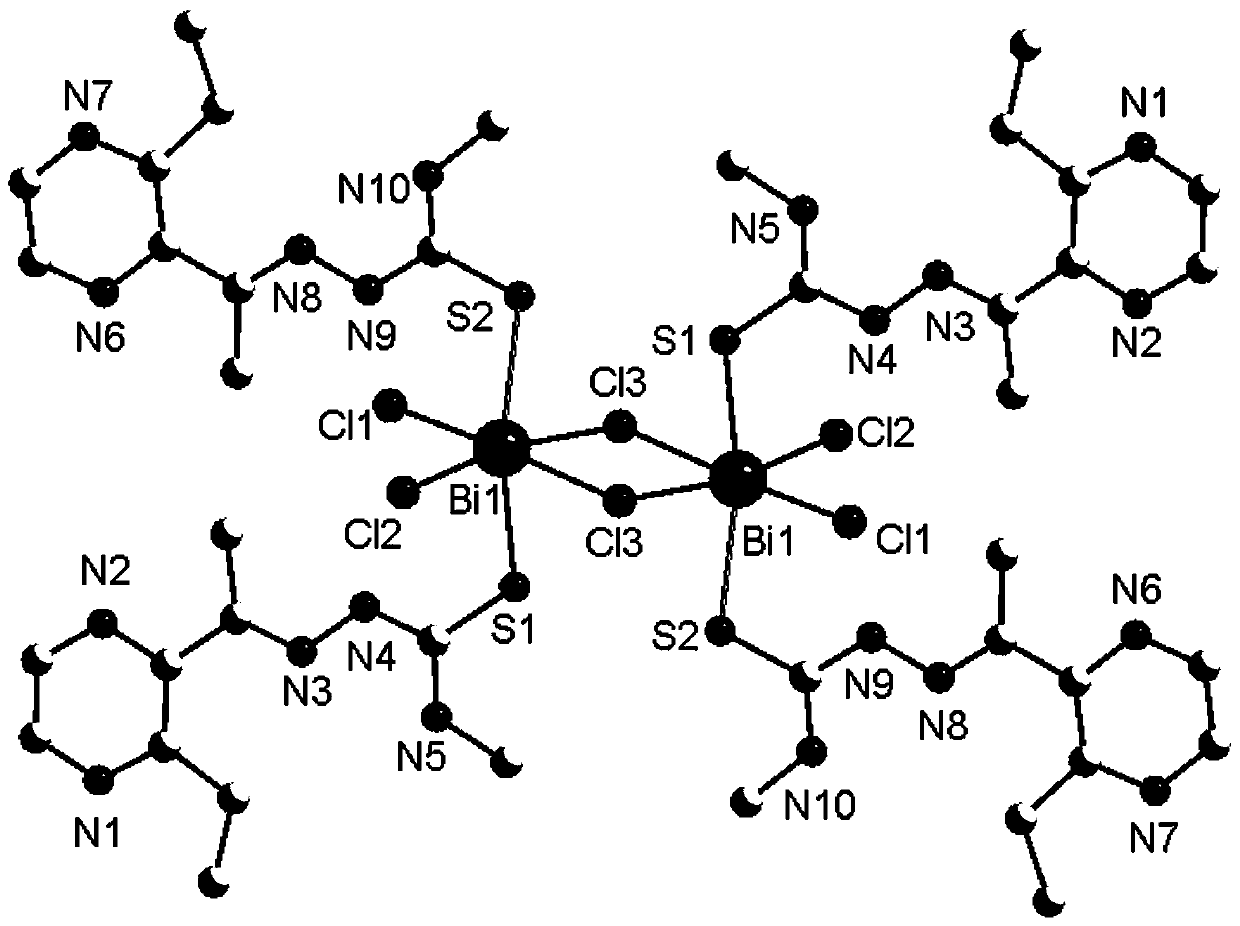
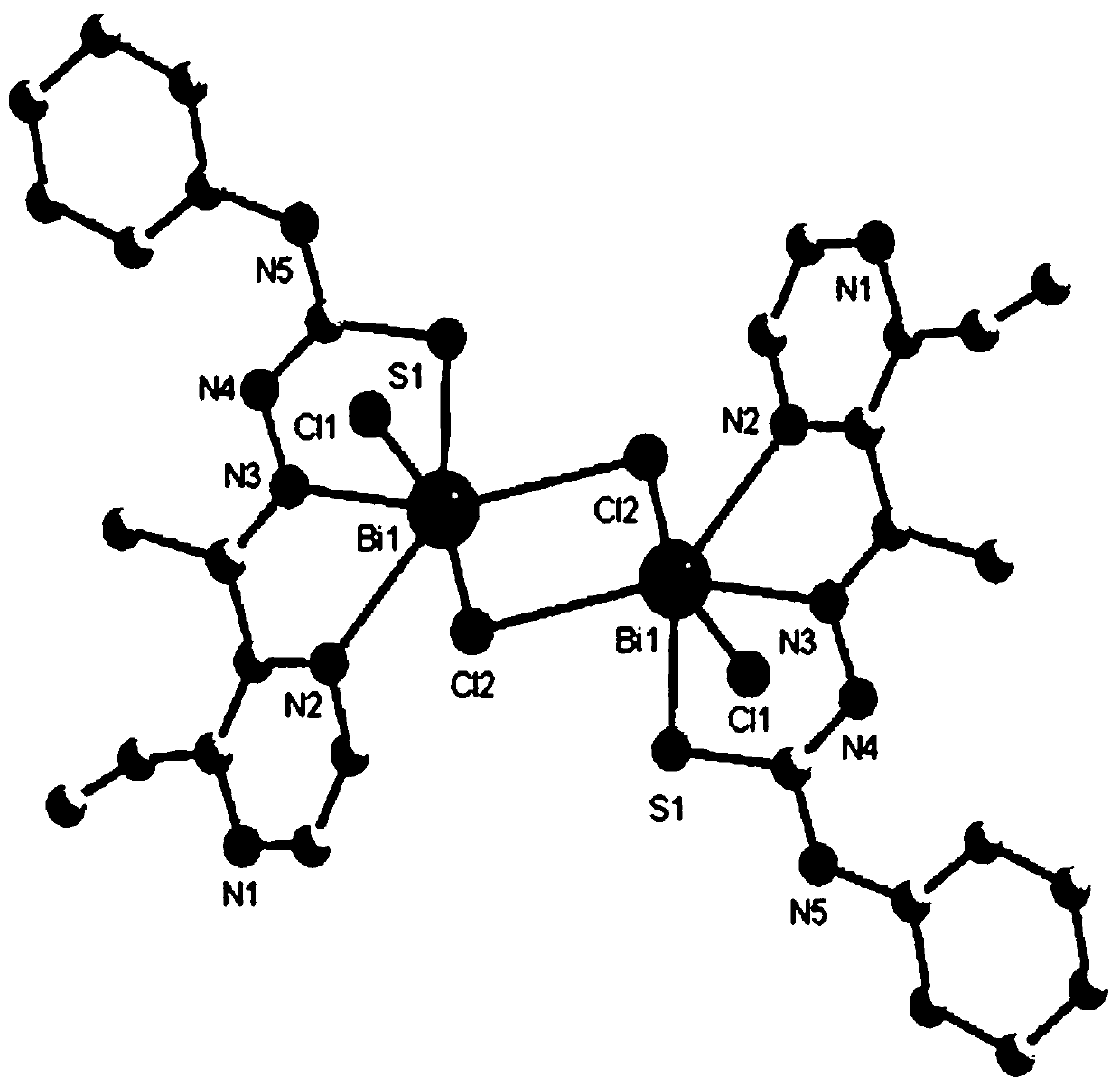
Bismuth compound with 2-acetyl-3-ethylpyrazine thiosemicarbazone as ligand and synthesis method of bismuth compound
A technology of ethylpyrazine thiosemicarbazone and thiosemicarbazone, applied in bismuth organic compounds, drug combinations, antineoplastic drugs, etc., can solve the problems of stimulation, serious side effects, cancer cell drug resistance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The synthesis of bismuth compound C1 comprises the following steps:
[0032] (1) Dissolve 10 mmol of 2-acetyl-3-ethylpyrazine in 20 ml of methanol, and stir at 60° C. for 15 min to obtain a solution;
[0033] (2) Take 10 mmol of thiosemicarbazide and dissolve it in 20 ml of methanol, then drop the solution prepared in step (1) into the methanol solution containing thiosemicarbazide drop by drop, and reflux and stir at 60°C for 24 hours to obtain a pale yellow precipitate After the reaction, it was cooled to room temperature and then poured into a beaker to volatilize. After filtering the obtained precipitate, it was washed 3 times with absolute ethanol, and after drying, the ligand L1 was obtained;
[0034] Yield: 79.6%, Anal. Calcd (%) for C 9 h 13 N 5 S:C,48.41;H,5.87;N,31.36;S,16.36.Found:C,48.37;H,5.80;N,31.43;S,16.40.IR,cm -1 :3428(s,amide),3237(s,NH),3167(m,aromatic hydrogen),1597(m),1502(s),1459(s,aromatic),1399(m,C=N),1290 (s,thioamide),1155(s),1100(s),880(...
Embodiment 2
[0038] The synthesis of bismuth compound C2 comprises the following steps:
[0039] (1) Dissolve 10 mmol of 2-acetyl-3-ethylpyrazine in 20 ml of methanol, and stir at 60° C. for 15 min to obtain a solution;
[0040] (2) Get 10mmol of 4-methylthiosemicarbazide and dissolve it in 20ml of methanol, then drop the solution prepared in step (1) dropwise into the methanol solution containing 4-methylthiosemicarbazide, at 60°C Reflux and stir the reaction for 24 hours to obtain a light yellow precipitate. After the reaction, cool to room temperature and pour it into a beaker for volatilization. After filtering the obtained precipitate, wash it with absolute ethanol three times and dry it to obtain the ligand L2;
[0041] Yield: 86.7%, Anal. Calcd (%) for C 10 h 15 N 5 S: C, 50.61; H, 6.37; N, 29.51; S, 13.51. Found: C, 50.66; H, 6.35; N, 29.54; (s,NH),2935(m,aromatic hydrogen),1639(m),1615(s),1566(s),1467(s,aromatic),1347(m,C=N),1270(s,thioamide ),1171(s),1082(s),1055(m),971(m,C-...
Embodiment 3
[0045] The synthesis of bismuth compound C 3 comprises the following steps:
[0046] (1) Dissolve 10 mmol of 2-acetyl-3-ethylpyrazine in 20 ml of methanol, and stir at 60° C. for 15 min to obtain a solution;
[0047] (2) Get 10mmol of 4-phenylthiosemicarbazide and dissolve it in 20ml of methanol, then drop the solution prepared in step (1) into the methanol solution containing 4-phenylthiosemicarbazide, at 60°C Reflux and stir the reaction for 24 hours to obtain a light yellow precipitate. After the reaction, cool to room temperature and pour it into a beaker for volatilization. After filtering the obtained precipitate, wash it three times with absolute ethanol and dry it to obtain the ligand L3;
[0048] Yield: 85.9%, Anal. Calcd (%) for C 15 h 17 N 5 S: C, 60.18; N, 23.39; H, 5.72; S, 10.71. Found: C, 60.17; N, 23.35; H, 5.74; (s,NH),2921(w,aromatic hydrogen),1610(m),1527(s),1379(s,aromatic),1354(s,C=N),1266(s,thioamide),1158(s ),1133(s),1064(m),980(m,C-H),669(m,C=S),62...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com