Channel synthesis method of m-triaminotrinitrobenzene
A technology of triaminotrinitrobenzene and trichlorotrinitrobenzene, which is applied in the channel synthesis field of m-triaminotrinitrobenzene, to achieve a wide reaction temperature, eliminate severity, and promote the effect of the reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
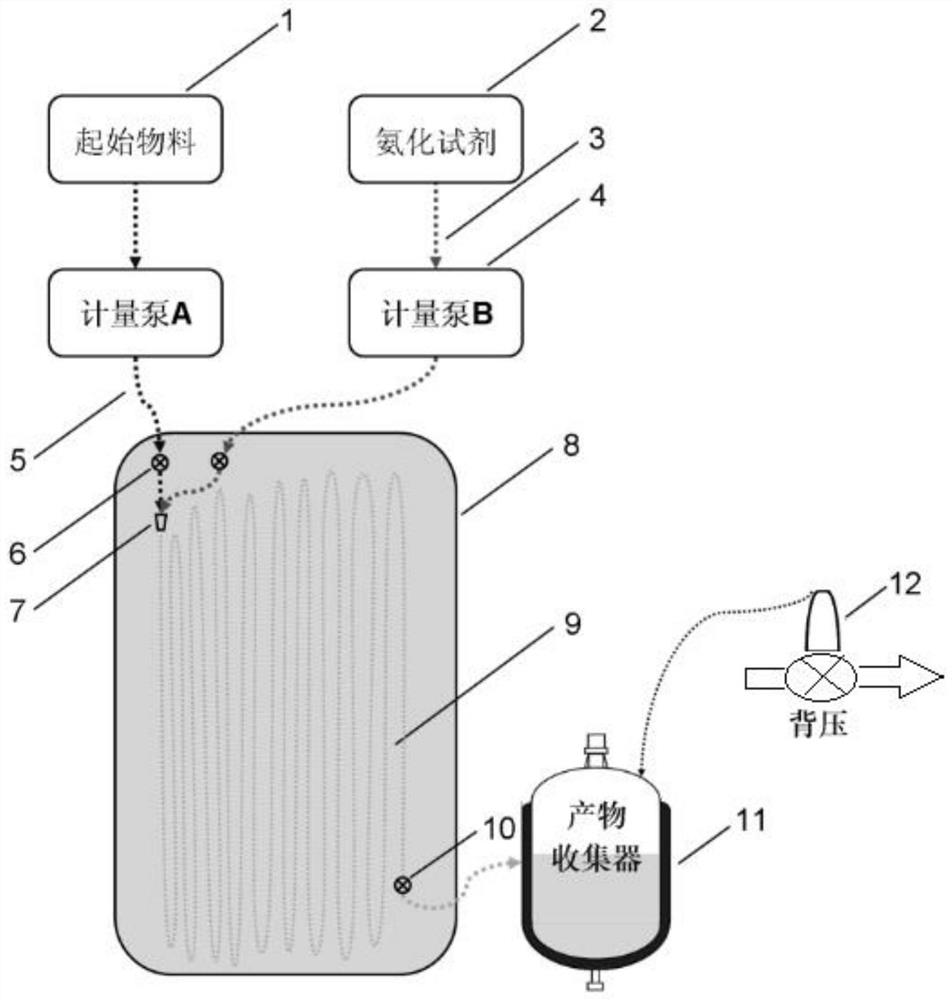
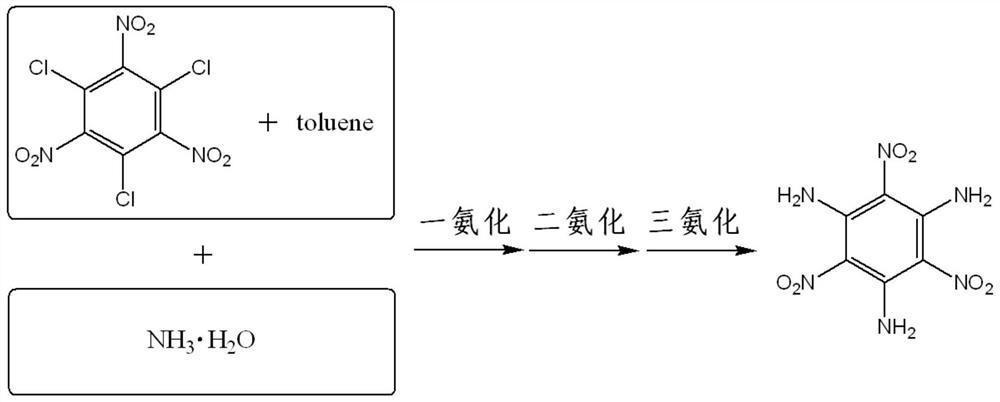
[0045]Weigh m-trichlorotrinitrobenzene (31.65g, 0.10mol) and toluene (316.5g, about 365.5ml) in a flask, stir and dissolve until the color is bright and there are no fine particles visible to the naked eye, as starting materials; weigh 25 % concentrated ammonia water (0.60mol, about 44.83ml) in the flask as the ammoniating reagent; start the metering pump to feed, and pump the starting material and the ammoniated reagent into the channel reactor, and the feeding rates of the two pumps are respectively set. are 2.4ml / min and 0.3ml / min, so that the molar ratio of m-trichlorotrinitrobenzene and ammonia water is approximately 1:6, and the pump pressure during operation is 0.01 to 0.95Mpa (the pump pressure threshold is set to 1.80Mpa); The residence time in the channel reactor was about 1 min, and the oil bath temperature was 70 °C; sampling was taken at the outlet of the microchannel reactor, and it was confirmed by liquid chromatography that part of the m-trichlorotrinitrobenzene...
Embodiment 2
[0047] Weigh m-trichlorotrinitrobenzene (31.65g, 0.10mol) and toluene (316.5g, about 365.5ml) in a flask, stir and dissolve until the color is bright and there are no fine particles visible to the naked eye, as starting materials; weigh 25 % concentrated ammonia water (0.90mol, about 67.25ml) in the flask, as the ammoniation reagent; start the metering pump feeding, pump the starting material and the ammoniated reagent into the channel reactor, and the feeding rates of the two pumps are respectively set to 1.6ml / min and 0.3ml / min, so that the molar ratio of m-trichlorotrinitrobenzene and ammonia water is close to 1:9, and the pump pressure during operation is 0.01-0.95Mpa (the pump pressure threshold is set to 1.80Mpa); the material is in the channel The residence time in the reactor was about 3min, and the temperature of the oil bath was 90°C; the reaction material flowed out from the channel reactor to the collector, and the reaction was continued for 0 to 5 hours; Analysis ...
Embodiment 3
[0049] Weigh m-trichlorotrinitrobenzene (31.65g, 0.10mol) and toluene (316.5g, about 365.5ml) in a flask, stir and dissolve until the color is bright and there are no fine particles visible to the naked eye, as starting materials; weigh 25 % concentrated ammonia water (0.90mol, about 67.25ml) in the flask, as the ammoniation reagent; start the metering pump feeding, pump the starting material and the ammoniated reagent into the channel reactor, and the feeding rates of the two pumps are respectively set to 4.8ml / min and 0.8ml / min, make the molar ratio of m-trichlorotrinitrobenzene and ammonia water close to 1:8, the pump pressure during operation is 1.3~1.5Mpa (the pump pressure threshold is set to 1.80Mpa, adjust the back pressure valve to maintain the material pressure in the reverse channel); the residence time of the material in the channel reactor is about 10min, the oil bath temperature is 110 °C, and ultrasonic assistance; sampling at the outlet of the channel reactor, c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com