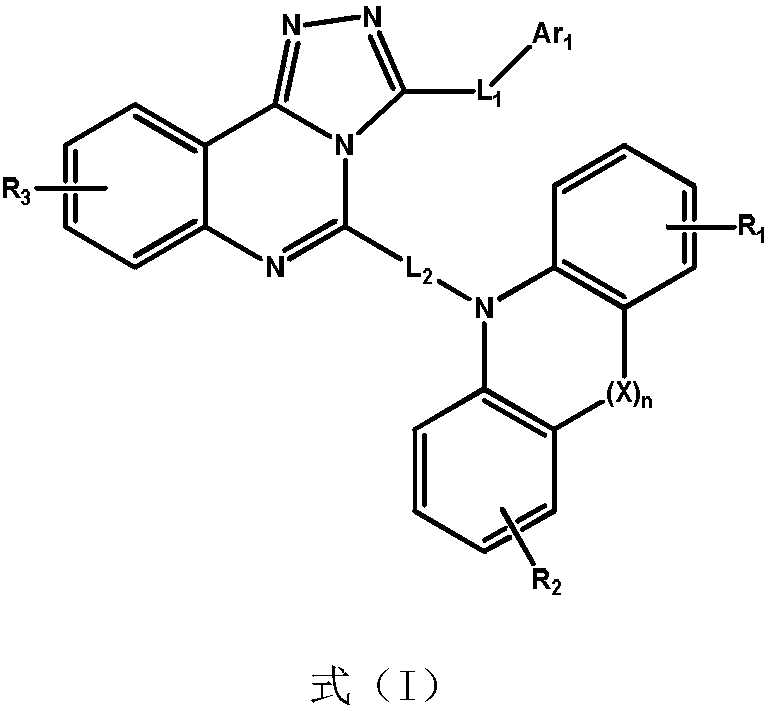
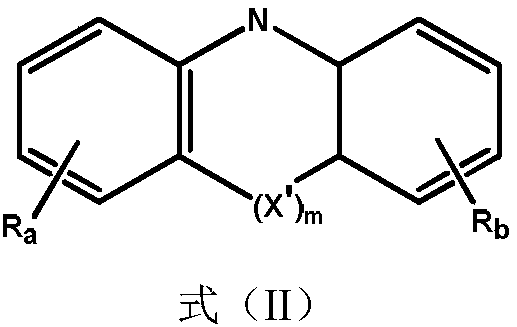
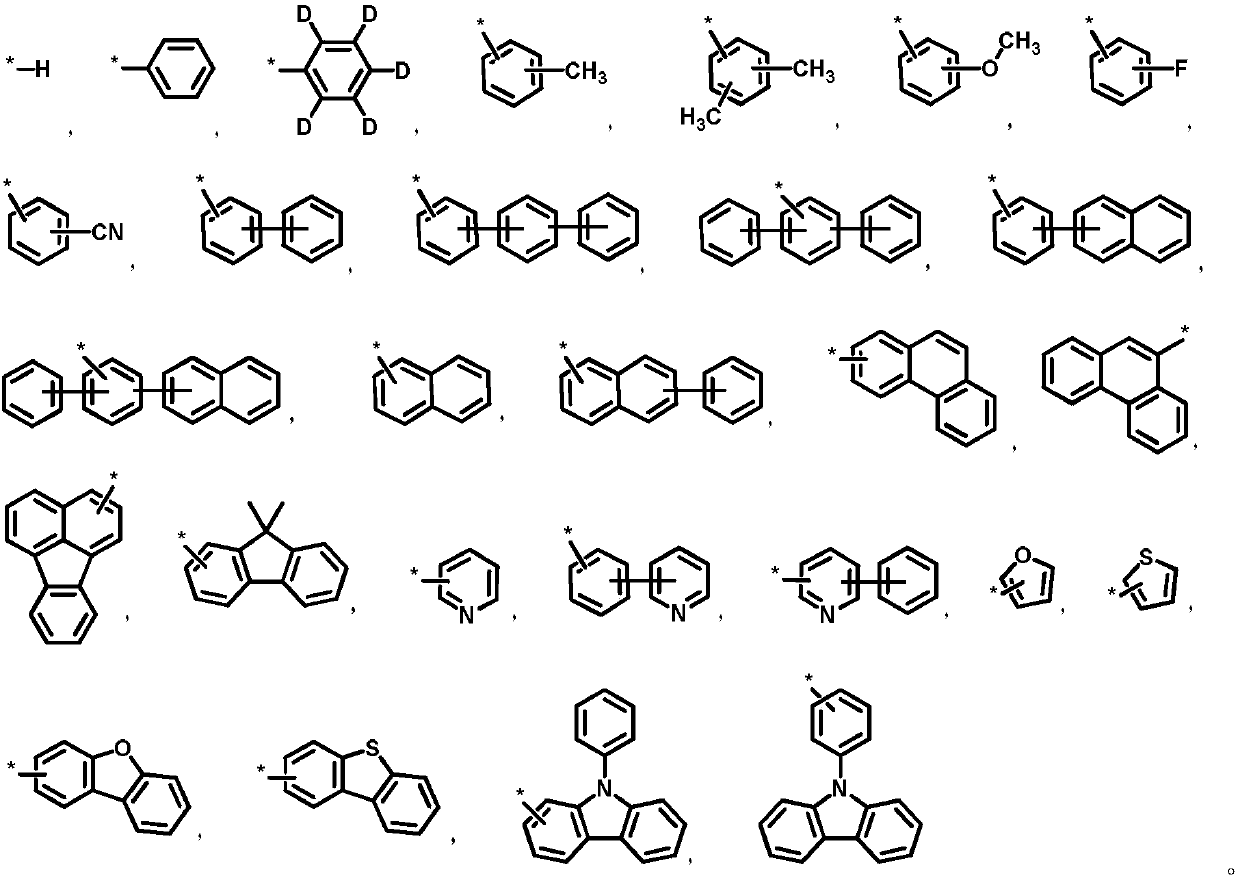
Quinazoline triazole derivative and application thereof in field of organic electroluminescence
A compound and unsubstituted technology, applied in the field of new organic heterocyclic compounds, can solve the problem of not specifically disclosing organic electroluminescent compounds, etc., and achieve the effects of wide energy gap, simple device structure, and improved energy level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Embodiment 1: the preparation of compound C1
[0055]
[0056] Preparation of Compound 1-1
[0057] 2,4-Dichloroquinazoline (500g, 2.5mol) was dissolved in a 10L ethanol flask, and hydrazine hydrate (470g, 7.5mol, 80% aqueous solution) was added dropwise at 5°C under stirring, and the temperature was kept below 10°C. After the dropwise addition, the mixture was naturally raised to room temperature for 1 hour, and the obtained solid was filtered with suction, washed with water and ethanol, and dried in the air to obtain compound 1-1 (415 g, 86%) as an off-white solid.
[0058] Preparation of compound 1-2 (reference J.Heterocyclic chem.27,497,1990)
[0059] Compound 1-1 (200g, 1.03mol) was added to a flask containing 2L of ethanol, and benzaldehyde (120g, 1.13mol) was added dropwise under stirring at room temperature. After the addition was complete, the stirring reaction was continued for 30 minutes, and the resulting solid was filtered and washed with ethanol and ...
Embodiment 2
[0066] Embodiment 2: the preparation of compound C2
[0067]
[0068] Preparation of compound 2-1
[0069] Compound 1-1 (20g, 103mmol) was added to a flask containing 200mL of ethanol, and phenyl-p-methylformaldehyde (15g, 125mmol) was added dropwise under stirring at room temperature. Rinse with n-hexane and dry to obtain compound 2-1 (19.8 g, 65%) as a yellow solid.
[0070] Preparation of compound 2-2
[0071] Compound 2-1 (19.8g, 67mmol) was added to a flask containing 200L of ethanol, and iodobenzene acetate (25.8g, 80.1mmol) was added in batches under stirring at room temperature. Stirring was continued for 1.5 hours after the addition, and TLC showed that the reaction was complete. After adding 200 mL of n-hexane and stirring for 5 minutes, the resulting solid was suction-filtered, rinsed with n-hexane, and dried as a light brown solid compound 2-2 (14.6 g, 74%).
[0072] Preparation of compound C2
[0073] Compound 2-2 (7.35g, 25mmol), compound 1-4 (10g, 23.64mm...
Embodiment 3
[0074] Embodiment 3: the preparation of compound C6
[0075]
[0076] Preparation of compound 3-1
[0077] Compound 1-1 (20g, 103mmol) was added to a flask containing 200mL of ethanol, and 3-formaldehyde pyridine (13.3g, 125mmol) was dropped under stirring at room temperature. Rinse with n-hexane and dry to obtain compound 3-1 (16.3 g, 56%) as a yellow solid.
[0078] Preparation of compound 3-2
[0079] Compound 3-1 (16.3g, 57.6mmol) was added to a flask containing 200L of ethanol, and iodobenzene acetate (22.3g, 69.1mmol) was added in batches under stirring at room temperature. Stirring was continued for 1.5 hours after the addition, and TLC showed that the reaction was complete. After adding 200 mL of n-hexane and stirring for 5 minutes, the obtained solid was suction-filtered, rinsed with n-hexane, and dried to obtain light brown solid compound 3-2 (10 g, 70%).
[0080] Preparation of compound C6
[0081] Add compound 2-2 (7.03g, 25mmol), compound 1-4 (10g, 23.64mmo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com