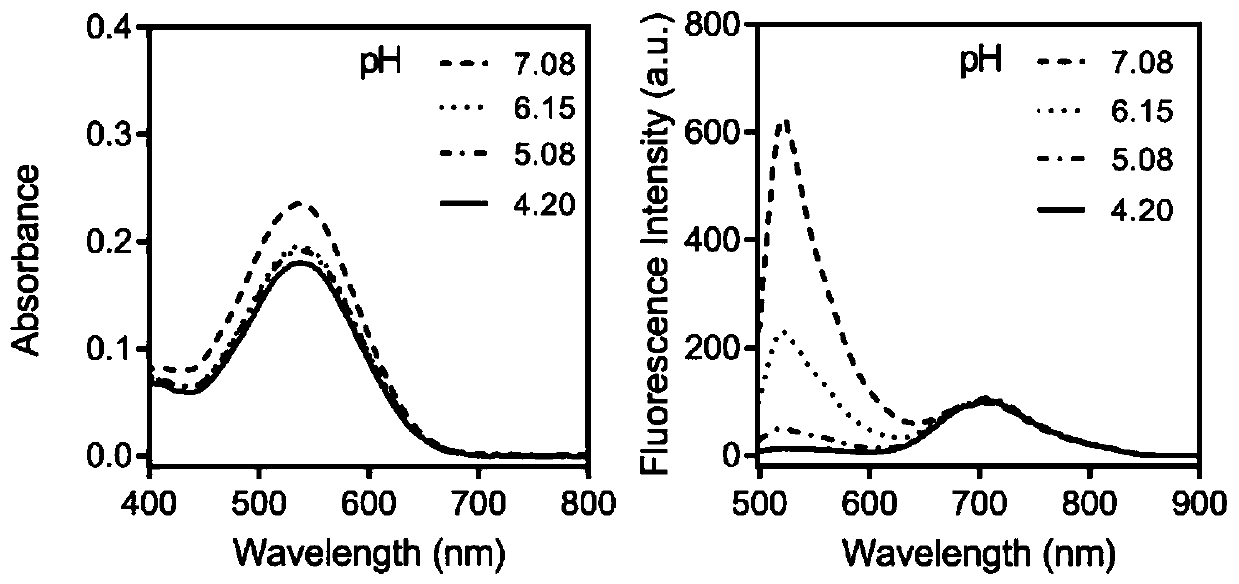
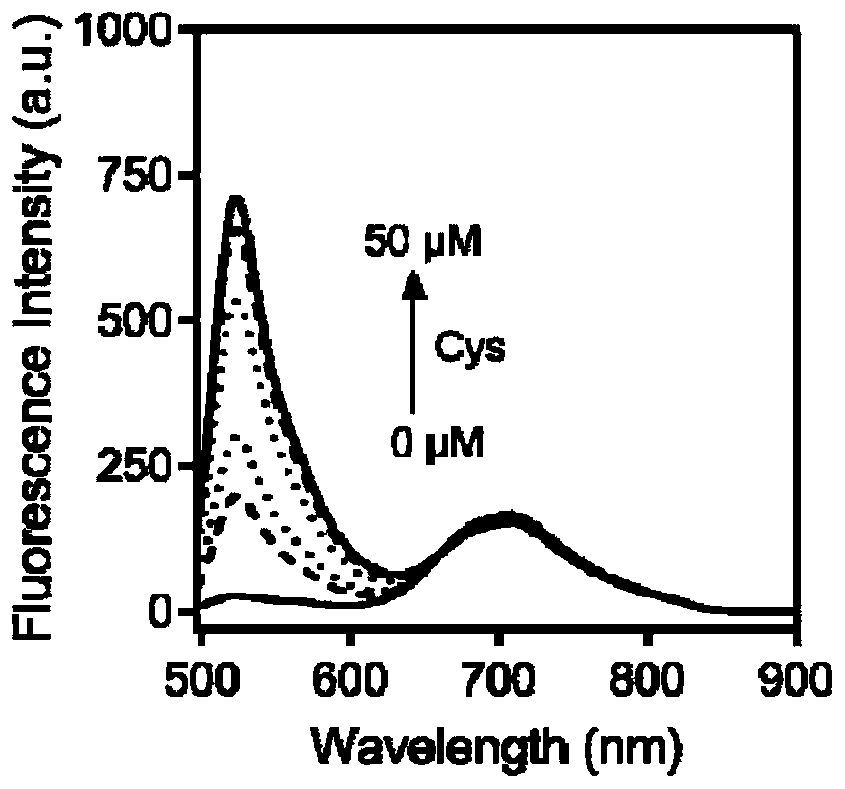
Internal standard ratio type fluorescent probe based on fluorescein derivative, preparation method and application thereof
A technology of fluorescent probes and derivatives, applied in the field of internal standard ratio fluorescent probes, can solve the problems of wrong fluorescence ratio, fluorescence ratio error, failure to provide, etc., and achieve the effect of simple synthesis and few synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] In the compound shown in formula I below, A is O; B is O; C is O or a group shown in II-3A; D is a group shown in formula III-1A or III-2A; E is H as an example, The method for synthesizing the compound shown in formula I is further elaborated, and the synthetic method comprises the following steps: it uses fluorescein as a starting material, successively undergoes Vilsmeier-Haack reaction, reacts with halogenated unsaturated hydrocarbon and reacts with active subunit containing Different conjugated groups of the methyl group undergo condensation reactions to obtain the target compound.
[0054] The present invention is further elaborated below by example, and its purpose is only to understand content of the present invention better. Therefore, the examples given do not limit the protection scope of the present invention:
Embodiment 1
[0056]
[0057] In a 100mL three-neck flask, add DMF (15mL), POCl 3 (0.5 mL) and fluorescein (4 g, 12 mmol). After 48 hours of reaction, the reaction was terminated, and 0.5 g of a yellow solid was obtained by recrystallization from acetone, with a yield of 11.2%.
[0058] 1 H-NMR (400MHz, DMSO-d 6 , ppm): δ6.61(s, 1H), 6.62(d, J=1.2Hz, 1H), 6.72(d, J=8.9Hz, 1H), 6.85(d, J=1.2Hz, 1H), 6.96 (d, J=8.9Hz, 1H), 7.32(d, J=7.5Hz, 1H), 7.74(t, J=7.5Hz, 1H), 7.82(t, J=7.5Hz, 1H), 8.03(d , J=7.5Hz, 1H), 10.26(s, 1H, -OH), 10.64(s, 1H, -CHO) 11.89(s, 1H, -OH).
[0059] Mass spectrometry (ESI negative ion mode for [M-H] - ): calcd for [C 21 h 11 o 6 ]: 359.0556; found: 359.0555.
[0060]
[0061] In a 100 mL three-neck flask, add fluorescein aldehyde (500 mg, 1.39 mmol) and acetic anhydride (50 mL). Add 2-Bromocyclohex-1-ene-1-carbaldehyde (523mg, 2.78mmol) into a three-necked flask and react at room temperature for 2h. During the course of the reaction, the color gradua...
Embodiment 2
[0069]
[0070] Add fluorescein derivative (50 mg, 0.078 mmol), dichloromethane (15 mL), and acryloyl chloride (0.5 mL) into a 50 mL three-necked flask. After reacting at room temperature for 18 h, the reaction was terminated. The solvent was spin-dried, and recrystallized from dichloromethane to obtain 10 mg of a black-purple solid with a yield of 18%.
[0071] 1 H-NMR (400MHz, CDCl 3 , ppm): δ1.91 (m, 2H, -CH 2 ), 2.52(t, 2H, -CH 2 ), 2.68(t, 2H, -CH 2 ), 6.08(d, J=10.44Hz, 1H, alkene-H), 6.15(d, J=15.52Hz, 1H, alkene-H), 6.34(dd, J 1 =10.48Hz,J 2=17.32Hz, 1H, alkene-H), 6.64(d, J=8.68Hz, 1H, Ph-H), 6.65(d, J=17.32Hz, 1H, alkene-H), 6.77(s, 1H, alkene -H), 6.79(d, J=8.92Hz, 1H, Ph-H), 6.83(s, 1H, Ph-H), 6.84(d, J=1.8Hz, 1H, Ph-H), 7.04(s , 1H, alkene-H), 7.19 (d, J = 7.4Hz, 1H, Ph-H), 7.25 (d, J = 1.8Hz, 1H, Ph-H), 7.40 (t, J = 7.4Hz, 1H , Ph-H), 7.52 (d, J=7.68Hz, 1H, Ph-H), 7.65-7.73 (m, 3H, Ph-H), 8.04 (d, 1H, J=15.52Hz, alkene-H) , 8.07(d, J=6.80Hz, 1H, Ph-H...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com