A Porcine Circovirus Type 2 Virus Strain and Its Application
A porcine circovirus, type 2 virus technology, applied in the direction of antiviral agents, virus/phage, virus antigen components, etc., can solve the mixed infection and secondary infection of multiple bacteria and viruses, economic losses in the breeding industry, and decreased body resistance and other problems, to achieve the effect of good vaccine immunization, strong product competitiveness, and prevention of epidemic and transmission.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
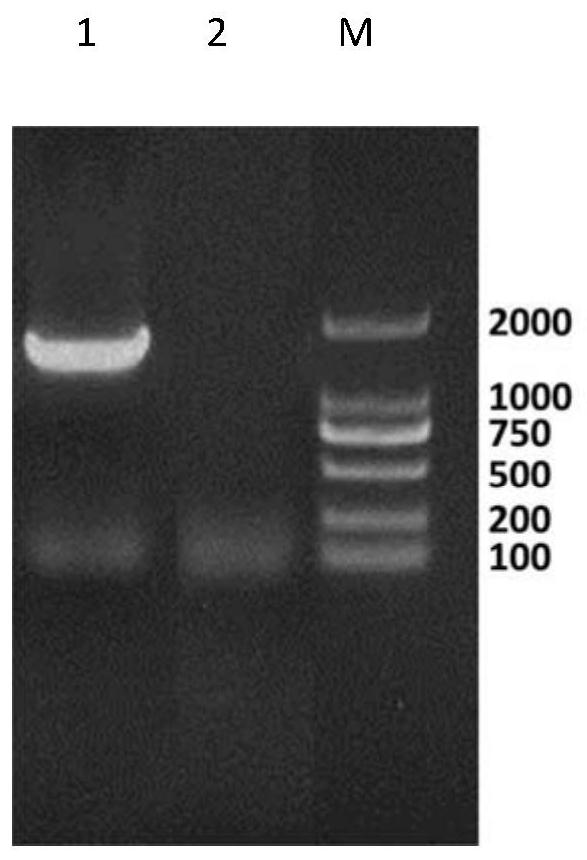
[0029] Example 1: Isolation and identification of porcine circovirus type 2 ZM-PCV2-13CGMCC No.17291.
[0030] 1.1 Experimental materials
[0031] The disease samples used in the experiment came from pig farms with porcine circus disease in Fujian Province, and the collection time was October 2017; the porcine kidney cell line (Porcine Kidney, PK-15) was purchased from ATCC, the American Culture Center.
[0032] 1.2 Primer design
[0033] Referring to the PCV2MF616427.1 gene series published in GenBank, two pairs of specific primers were designed using Primer5, and the primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. The nucleotide sequence of the specific primer is as follows:
[0034] PCV2-F-1: 5'-ACCAGCGCACTTCGGCAG-3';
[0035] PCV2-R-1:5'-AATACTTACAGCGCACTTC-3';
[0036] PCV2-F-2: 5'-TCACTTAGGGTTAAGTGG-3';
[0037] PCV2-R-2: 5'-AATGGCATCTTCAACACC-3';
[0038] 1.3 Collection and processing of disease materials
[0039] Collect samples of lymph n...
Embodiment 2
[0055] Embodiment 2: the mensuration of porcine circovirus type 2 virus strain ZM-PCV2-13 virus titer
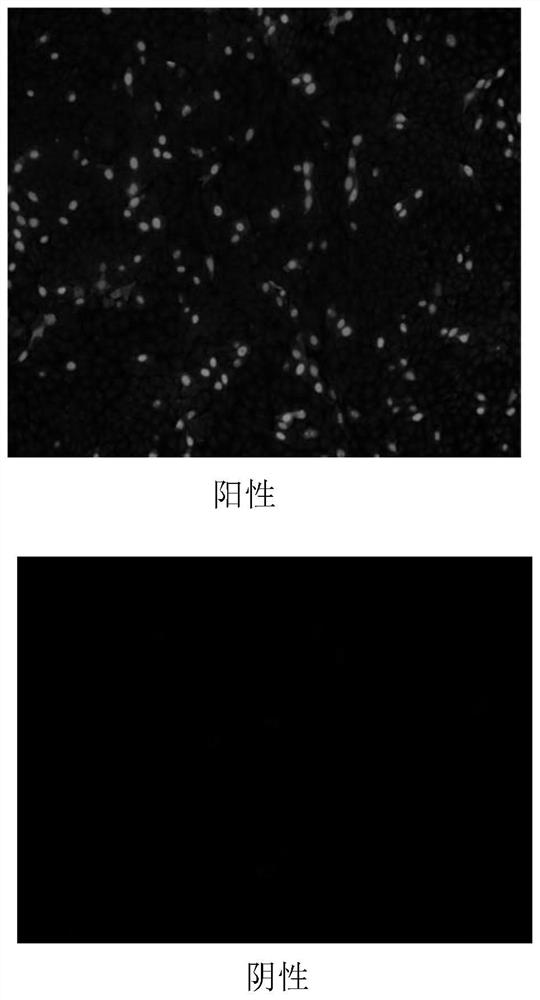
[0056] The isolated virus liquid was inoculated on the PK15-B1 cell monolayer, adsorbed at 37°C for 30 minutes, added DMEM cell maintenance solution containing 2% calf serum, cultured at 37°C for 48h, continued to pass to the 25th passage, and stored at -20°C. Use DMEM cell maintenance solution to make PCV2 10 -1 ~10 -8 Serially dilute and inoculate a monolayer of PK15-B1 cells in a 96-well plate, inoculate 4 wells for each dilution, and inoculate 100 μl in each well. At the same time set up a negative control, put CO 2 Cultivate at 37°C for 12h in an incubator, treat with 300mM D-glucosamine hydrochloride, and continue to cultivate at 37°C for 48h. Fix the cells with absolute ethanol, measure the number of wells containing green fluorescent substance cells in each dilution by the IFA method, and finally calculate the TCID of the virus by the Reed-Muench method 50 . The...
Embodiment 3
[0057] Embodiment 3: the preparation of porcine circovirus type 2 ZM-PCV2-13 strain inactivated vaccine
[0058] 3.1 Virus culture
[0059] Use low-sugar DMEM medium containing 10% fetal bovine serum, 100U / ml penicillin, 100g / ml streptomycin, T225cm 2 Cell culture flask, 37°C, 5% CO 2 Cultivate PK-15 cells adherently in an incubator; when the fusion degree reaches about 80%, discard the culture medium, inoculate porcine circovirus type 2 ZM-PCV2-13 strain, and then use 2% fetal bovine serum, 100U / ml penicillin, 100g / ml streptomycin low-sugar DMEM medium, cultured at 37°C, 5% CO2 for 12h, treated with 300mM D-glucosamine hydrochloride, continued to culture at 37°C for 48h, and harvested the virus culture; freeze-thawed repeatedly Centrifuge 3 times at 10000×g for 10 min to remove cell debris and collect the supernatant to obtain porcine circovirus type 2 virus liquid, which is stored at -80°C for later use.
[0060] 3.2 Inactivation of virus liquid and inactivation test
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| sedimentation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com