Preparation method of manganese-based composite metal oxide ozonolysis catalyst
A compound metal and ozone decomposition technology, applied in the direction of metal/metal oxide/metal hydroxide catalyst, physical/chemical process catalyst, separation method, etc., can solve the problem of short catalyst life, achieve high stability, and synthesis process The effect of simple, high catalytic deozone activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
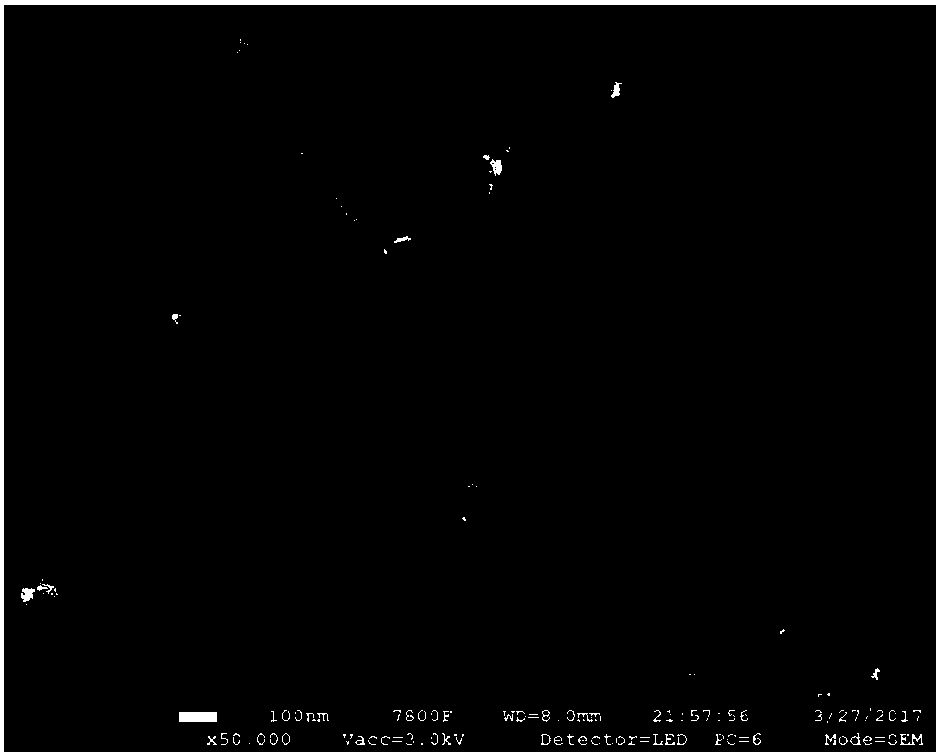
[0032] Dissolve manganese nitrate and cerium nitrate in water respectively, configure a solution with a Mn / Ce molar ratio of 19:1 and mix evenly, mix 50mL NaOH solution and 20mL 30% H 2 o 2 The solution is added to the mixed solution at the same time, the metal ion M n+ with OH - The molar ratio of the solution is 1:2, and the solution changes from a colorless transparent solution to a dark brown turbid solution. After stirring for 20 minutes, it is heated to 60°C in a water bath, refluxed for 12 hours, filtered with suction, washed with deionized water and absolute ethanol, and finally in Dry in a vacuum oven at 60°C for 12 hours, and then bake the vacuum-dried product at 350°C in an air atmosphere to obtain a nanofibrous catalyst CeMn 19 o x -NO 3 ( figure 1 ). The catalyst was pressed into tablets and sieved into 40-60 mesh particles, and 0.1 g of the catalyst was taken and placed in a tubular fixed-bed reactor for evaluation of the catalyst. The results are shown in...
Embodiment 2
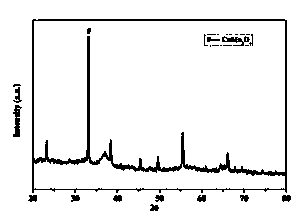
[0034] Dissolve manganese acetate and copper acetate in water respectively, configure a solution with a Mn / Cu molar ratio of 9:1 and mix evenly, mix 50mL NaOH solution and 20mL 30% H 2 o 2 The solution is added to the mixed solution at the same time, the metal ion M n+ with OH - The molar ratio of the solution is 1:2, and the solution changes from a blue transparent solution to a black turbid solution. After stirring for 20 minutes, it is heated to 60°C in a water bath, refluxed for 12 hours, filtered with suction, washed with deionized water and absolute ethanol, and finally in a vacuum Dry in an oven at 60°C for 12h. The vacuum-dried product was calcined at 350°C in an air atmosphere to obtain the catalyst CuMn 9 o x -Ac, the XRD pattern of the catalyst obtained is shown in figure 2 . XRD characterization shows that there is no obvious CuO peak, and CuMn is formed 2 o 4 composite metal oxides. The catalyst was pressed into tablets and sieved into 40-60 mesh particl...
Embodiment 3
[0036] Dissolve manganese nitrate and copper nitrate in water respectively, configure a solution with a molar ratio of Mn / Cu of 9:1 and mix evenly, mix 100mL NaOH solution and 30mL 30% H 2 o 2 The solution is added to the mixed solution at the same time, and the metal ion M n+ with OH - The molar ratio of the solution is 1:2, and the solution changes from a blue transparent solution to a black turbid solution. After stirring for 20 minutes, it is heated to 60°C in a water bath, refluxed for 12 hours, filtered with suction, washed with deionized water and absolute ethanol, and finally in a vacuum Dry in an oven at 60°C for 12h. The vacuum-dried product was calcined at 350°C in an air atmosphere to obtain a nanofibrous catalyst MnCu 9 o x -NO 3 ( image 3 ). The catalyst was pressed into tablets and sieved into 40-60 mesh particles, and 0.1 g of the catalyst was taken and placed in a tubular fixed-bed reactor for evaluation of the catalyst. The results are shown in Table...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com