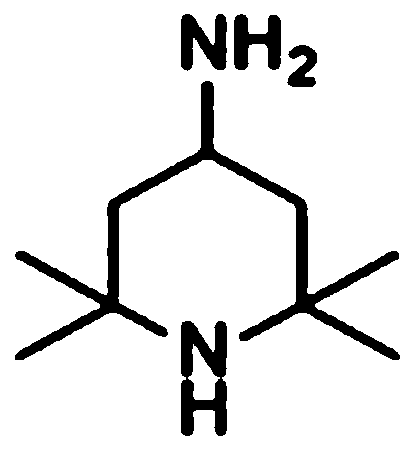
Synthetic method of amino-2,2,6,6-tetramentylniperidine with specific pH range
A technology of tetramethylpiperidinium synthesis method, applied in the field of chemical industry, can solve the problems of not adjusting the pH of the reaction system, not conforming to the concept of green chemistry, and high economic cost, so as to reduce ring-opening products, mild reaction conditions, reduce The effect of reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
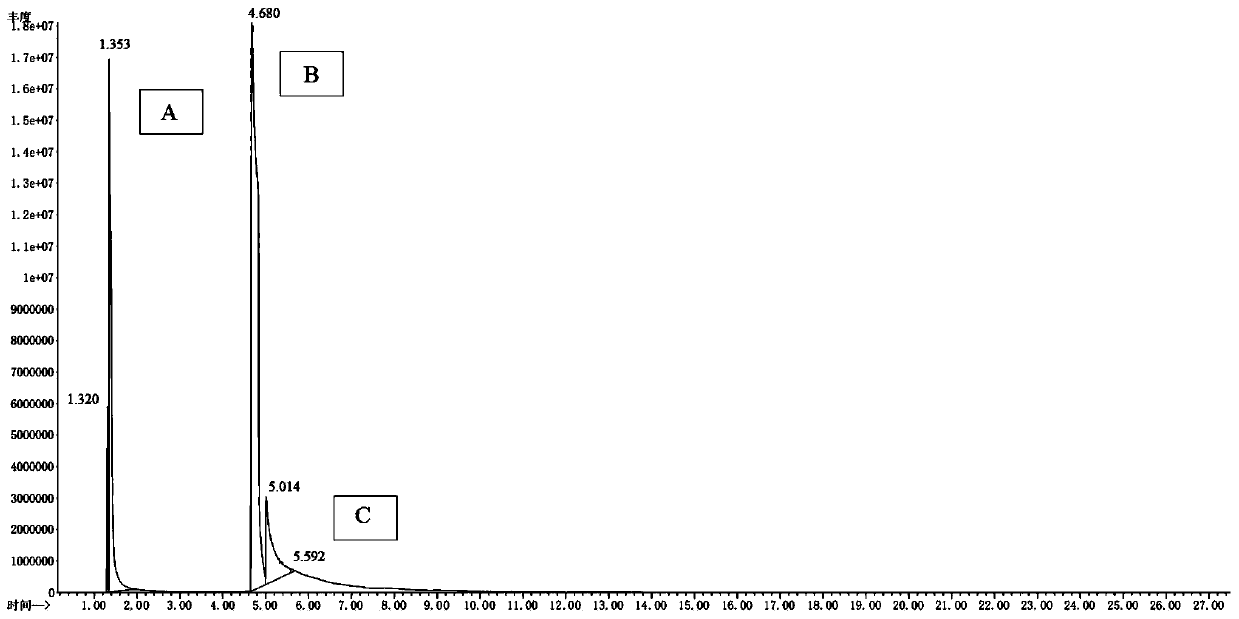
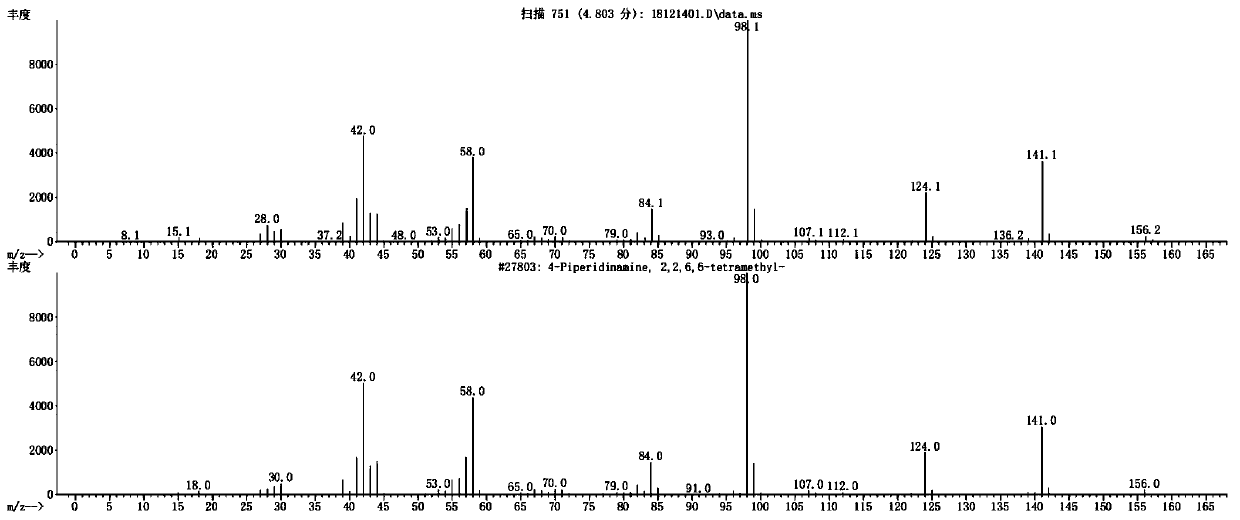
Image
Examples
Embodiment 1
[0038] Embodiment 1, a synthesis method of 2,2,6,6-tetramethylpiperidinamine in a specific pH range, the following steps are carried out in sequence:
[0039] (1) Add 30ml of water into the autoclave, 10g (0.0644mol) of raw material 2,2,6,6-tetramethylpiperidone, 15g (0.22mol of ammonia) with a mass fraction of 25%, add NaOH for about 0.05g, thereby adjusting the pH value of the system=12.0, and then adding 0.5g framework nickel as a catalyst.
[0040] (2) feed nitrogen to replace the air in the still, and after nitrogen replacement, feed hydrogen to replace the nitrogen in the still, and now the pressure in the still is 0Mpa. Turn on the stirring and heating device, so that the material in the kettle is raised to 60°C, and the stirring speed is 400 rpm.
[0041] (3) After stirring for 2 hours, start to feed hydrogen into the kettle until the pressure is 2.5MPa, and keep it warm for reaction; when the hydrogen in the kettle drops to ≤1.5MPa, continue to feed hydrogen until th...
Embodiment 2
[0044]Example 2. In Example 1, "add about 0.05 g of NaOH" is changed to "add about 0.1 g of NaOH". At this time, the pH of the system is 12.7, and the rest are the same as in Example 1. The product yield was 96.2%, and the purity was 99.1%.
[0045] Explanation: Add a small amount of NaOH appropriately to adjust the pH of the system to 13; the rest are the same as in Example 2 above; the yield and purity of the obtained product are basically the same as in Example 2.
Embodiment 3
[0046] Embodiment 3. In embodiment 1, "make the material in the kettle rise to 60°C" is changed to "raise the temperature to 55°C". The rest are equal to Example 1.
[0047] The product yield was 96.1%, and the purity was 99.1%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com