Antibody Adjuvant Conjugates
A technology of immunoconjugates and antibodies, applied in the field of immunoconjugates, can solve problems such as the inability of the immune system to recognize new antigens
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[1029]
[1030]
[1031] wherein X is halogen (eg, iodine, bromine, or chlorine); R' is H or sulfo; R" is optionally substituted aryl (eg, 3-carboxy-4-nitrophenyl) or optionally substituted Substituted heteroaryl (eg, pyridin-2-yl); R"' is optionally substituted alkyl (eg, methoxy); Z 1 ,Z 2 ,Z 3 and Z 4 as above; and the dotted line Indicates the point of attachment of the adjuvant moiety.
[1032] In some embodiments, the linker moiety-Z 1 -Z 2 -Z 3 -Z 4 -Z 5 -for:
[1033]
[1034] where dotted line indicates the point of attachment of the adjuvant moiety, and the wavy line Indicates the point of attachment of the amino acid side chain in the antibody. In some such embodiments, the amino acid side chain is a cysteine side chain or a modified lysine side chain containing a thiol group.
[1035] In some embodiments, the immunoconjugate has a structure according to Formula III:
[1036]
[1037] or a pharmaceutically acceptable salt thereof, wher...
Embodiment 1
[1489] Example 1: Imidazoquinolines for Antibody Conjugation
[1490] Synthesis of imidazoquinoline compounds with free amine groups (compound 1) or maleimide groups (compound 2) according to Scheme 1 allowed rapid assessment of linker technology and antibody-adjuvant immunoconjugate efficacy.
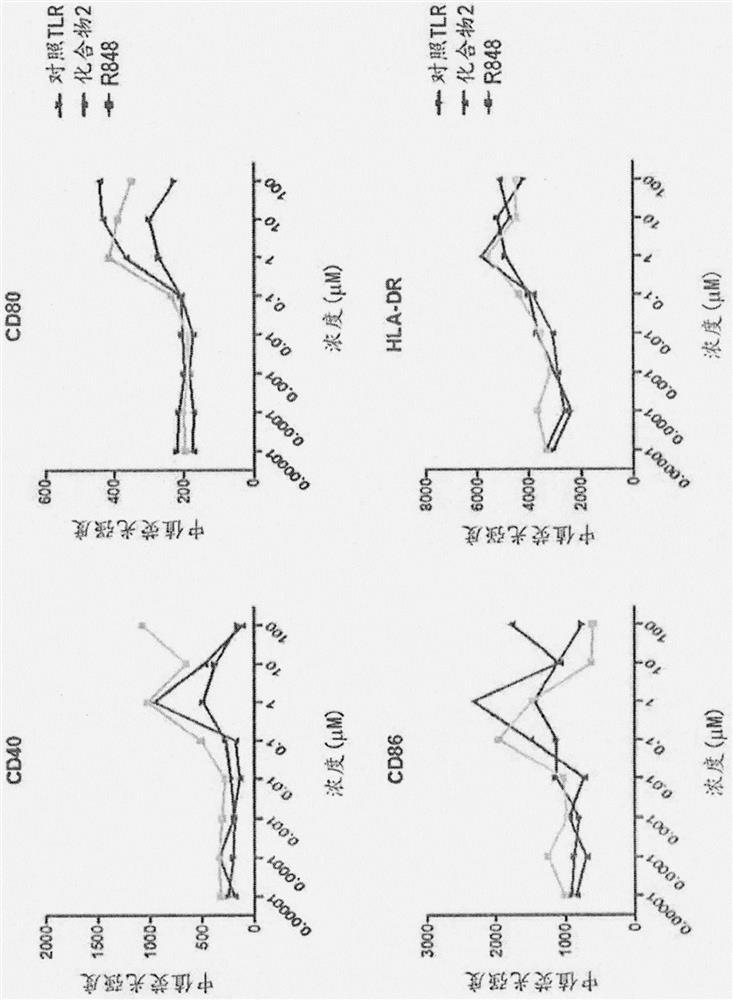
[1491] To determine whether adjuvant functionalization affects the ability of Compound 2 or Compound 1 to elicit immune activation prior to analysis via flow cytometry. Human antigen-presenting cells were stimulated with 10-fold serial dilutions of R848, compound 2, compound 1 or the control TLR agonist, CL307, for 18 hours. The data indicated that Compound 2 and Compound 1 performed similarly to R848 at each concentration measured ( Figure 4 ; compound 1 data not shown).
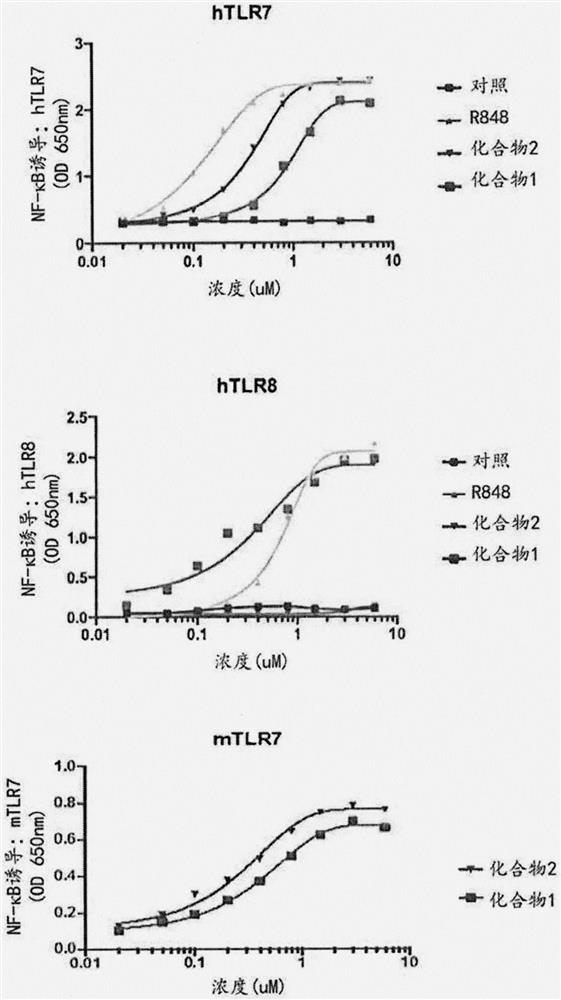
[1492] Afterwards, the ability of each functionalized TLR agonist to activate human TLR7 or TLR8 was directly assayed. HEK293 cells were co-transfected with human TLR7 or TLR8 or murine TLR7 and an inducible s...
Embodiment 2
[1495] Example 2: Preparation of antibody adjuvant conjugates
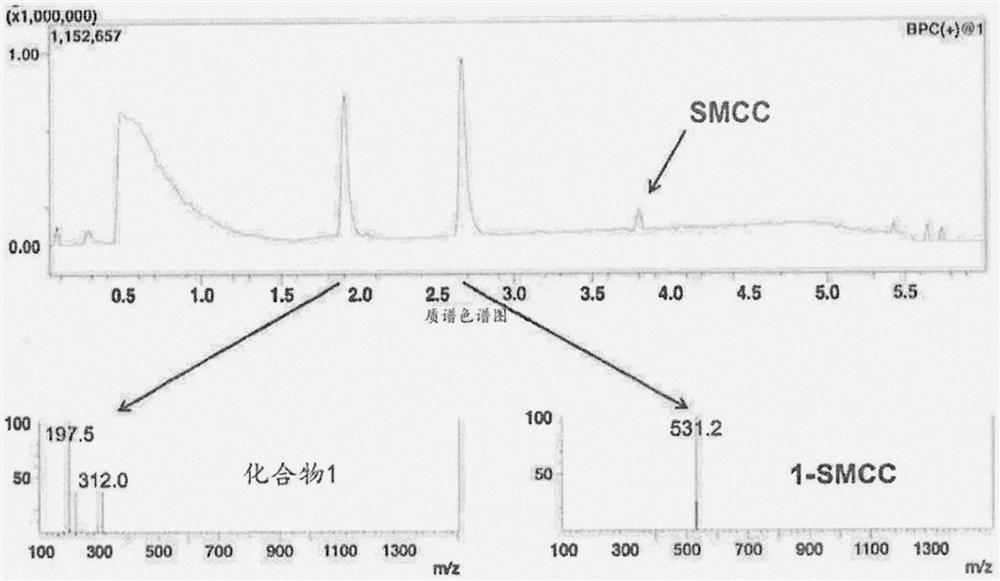
[1496] According to the general protocol outlined in Scheme 2A and Scheme 2B, compound 1 was modified with a non-cleavable crosslinker (SMCC, ThermoFisher Scientific) and a cleavable crosslinker (SPDP, ThermoFisher Scientific) in preparation for combination with rituximab conjugate.
[1497] Scenarios 2A and 2B
[1498]
[1499] Adjuvants with free amines (R848, compound 1, etc.) were linked to SMCC, SPDP, or other NHS-containing compounds by reacting the compounds at a 1:1 molar ratio in PBS, pH 7-7.5, or other suitable buffer Subconjugation. All reactions were protected from light and incubated at room temperature for 30 min. Where possible, adjuvant-crosslinker conjugates were purified via reverse phase high performance liquid chromatography (HPLC). Adjuvant-crosslinker conjugates were used immediately after conjugation as described below.
[1500] The adjuvant-linker composition was desalted with Z...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com