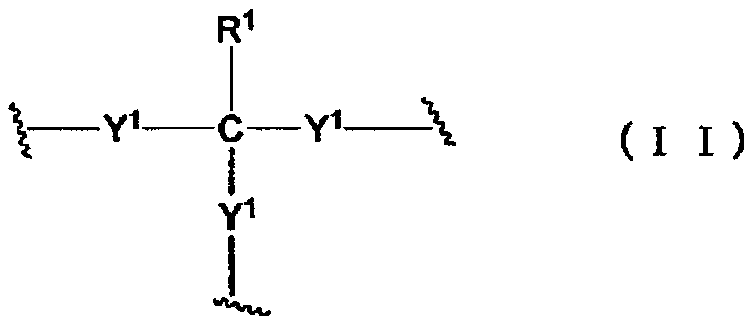
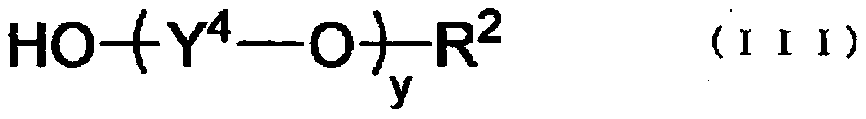Isocyanate composition, aqueous dispersion of isocyanate composition, production method therefor, coating composition, and coating film
An isocyanate and isocyanate-based technology, applied in the direction of polyurea/polyurethane coatings, coatings, etc., can solve the problems of reduced number of functional groups and reduced drying properties, and achieve the effect of excellent appearance of the coating film
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0340] Hereinafter, the present invention will be described in more detail based on examples and comparative examples, but the present invention is not limited to the following examples at all. In addition, unless otherwise specified, "part", "%" and "ppm" mean "parts by mass", "% by mass" and "ppm by mass", respectively.
[0341]
[0342] The NCO content (mass %) was determined by back titration with 1N hydrochloric acid after neutralizing the isocyanate groups in the measurement sample with an excess of 2N amine.
[0343]
[0344] Using the isocyanate composition as a sample, the average number of y was determined by proton nuclear magnetic resonance (NMR) using the following apparatus and conditions. Here, the average number of the alkylene glycol repeating units y in the isocyanate composition is obtained by correlating the integral value corresponding to the relative intensity of the alkylene group with the integral value corresponding to the relative intensity of the...
manufacture example 1
[0460] (Production Example 1) Synthesis of LTI
[0461] Put 122.2 g of ethanolamine, 100 mL of o-dichlorobenzene, and 420 mL of toluene into a four-neck flask equipped with a stirrer, a thermometer, and a gas inlet tube, and introduce ice-cooled hydrogen chloride gas to convert ethanolamine into hydrochloride. Next, 182.5 g of lysine hydrochloride was added, the reaction liquid was heated to 80° C. to dissolve ethanolamine hydrochloride, and hydrogen chloride gas was introduced to prepare lysine dihydrochloride. Furthermore, hydrogen chloride gas was passed through at a rate of 20 to 30 mL / min, the reaction solution was heated to 116° C., and the temperature was maintained until no more water was distilled out. The resulting reaction mixture was recrystallized from a mixture of methanol and ethanol to obtain 165 g of lysine β-aminoethyl ester trihydrochloride. Suspend 100 g of this lysine β-aminoethyl ester trihydrochloride in the form of a fine powder in 1200 mL of o-dichlor...
manufacture example 2
[0462] (Production Example 2) Synthesis of GTI
[0463] Put 275g of glutamic acid hydrochloride, 800g of ethanolamine hydrochloride, and 150mL of toluene into a four-necked flask equipped with a stirrer, a thermometer, and a gas inlet tube, and heat and reflux at 110°C for 24 hours while blowing hydrogen chloride gas. until the water is no longer azeotropic. The resulting reaction mixture was recrystallized from a mixture of methanol and ethanol to obtain 270 g of bis(2-aminoethyl)glutamate trihydrochloride. Suspend 85 g of the bis(2-aminoethyl) glutamate trihydrochloride in 680 g of o-dichlorobenzene, heat up the reaction solution while stirring, and start at a speed of 0.8 mol / hour when it reaches 135° C. Phosgene was blown in and maintained for 13 hours, and the reaction product was filtered, concentrated under reduced pressure, and purified by a thin film evaporator to obtain 54 g of GTI. The NCO content was 39.8% by mass.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| acid value | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com