Synthesis method of chiral benzocyclic beta-ketoester compounds
A synthesis method and compound technology are applied in the synthesis field of chiral benzocyclic β-ketoester compounds, can solve rare problems and the like, and achieve the effects of simple synthesis, mild reaction conditions, and cheap and easy-to-obtain catalysts
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
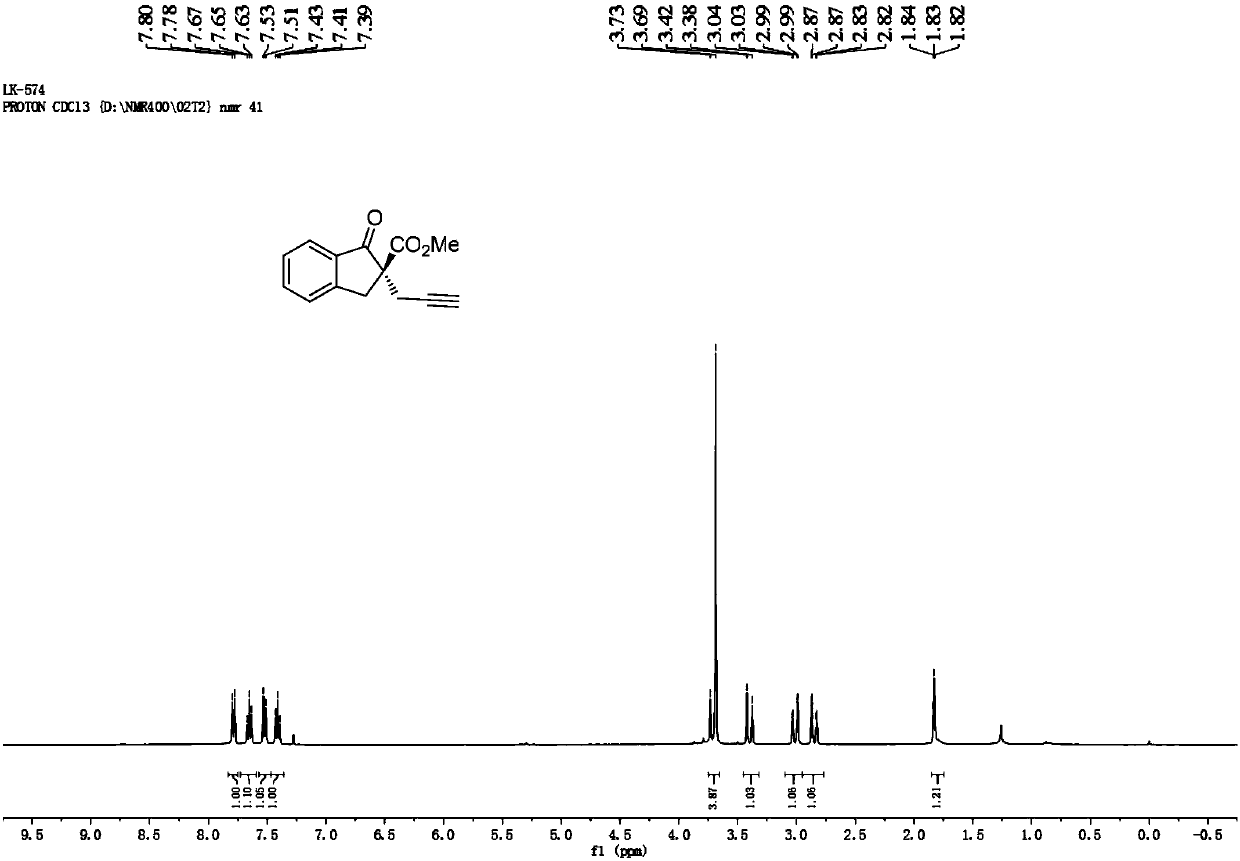
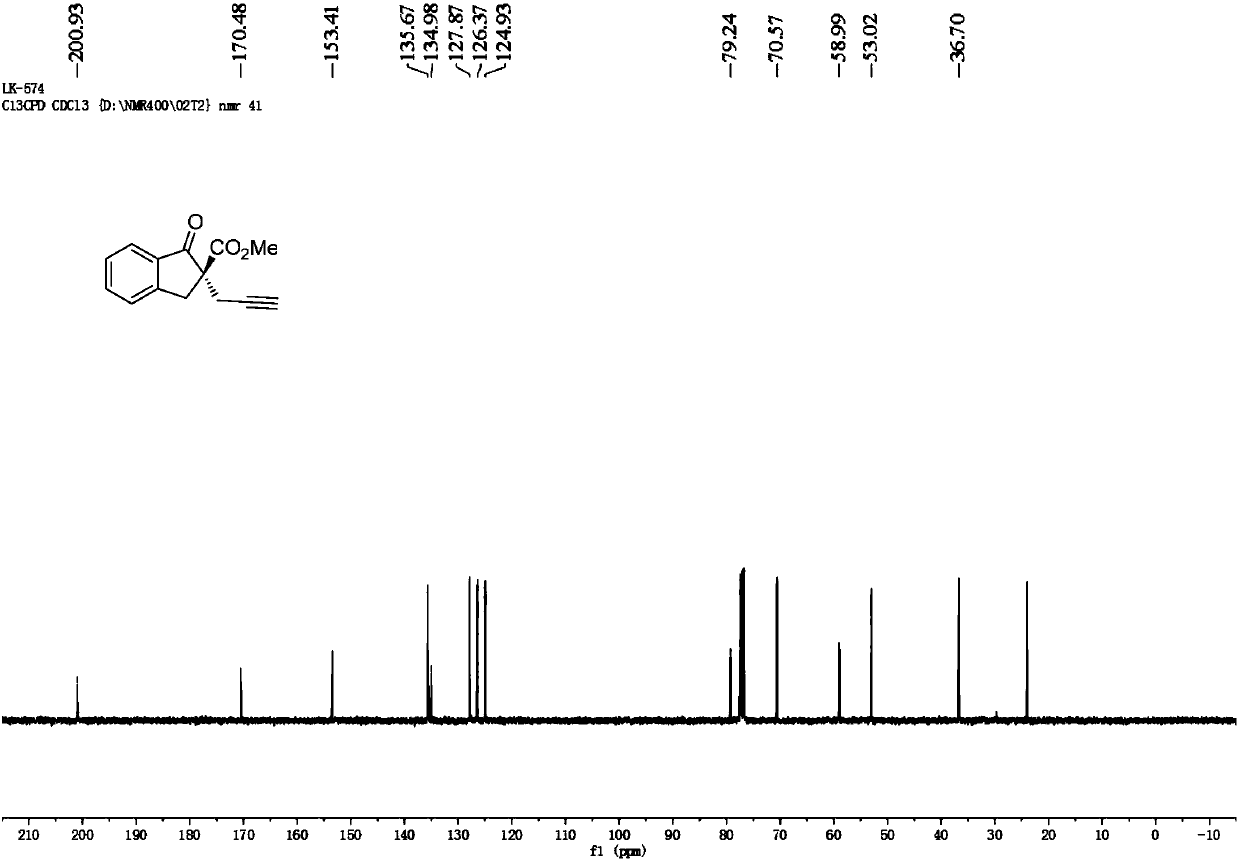
[0061] Cu(CH 3 EN) 4 BF 4 Complexation with L-2-1 is used as a catalyst to catalyze the reaction to generate 1-indanone-2-carboxylic acid methyl ester substituted product 2-propargyl-1-indanone-2-carboxylic acid methyl ester Ⅰ-1.
[0062] Add the metal precursor Cu(CH 3 EN) 4 BF 4(0.01 mmol, 5 mol %) and chiral ligand L-2-1 (0.012 mmol, 6 mol %), add 1.0 mL of anhydrous methanol under nitrogen protection, and stir at room temperature for 1 hour. Then the reaction tube was moved to a constant temperature reaction refrigerator at -20 °C, and propargyl alcohol ester IV-1 (0.2 mmol, 1.0 equiv), 1-indanone-2-carboxylic acid methyl ester III-1 (0.4 mmol, 2.0 equiv) equiv) and i Pr 2 NEt (0.4 mmol, 2.0 equiv) was dissolved in 2.0 mL of anhydrous methanol, then the solution was added to the above stirred catalyst solution under nitrogen protection, and the reaction was stirred at -20 °C for 12 h. After the reaction was completed, it was concentrated under reduced pressure unti...
Embodiment 2
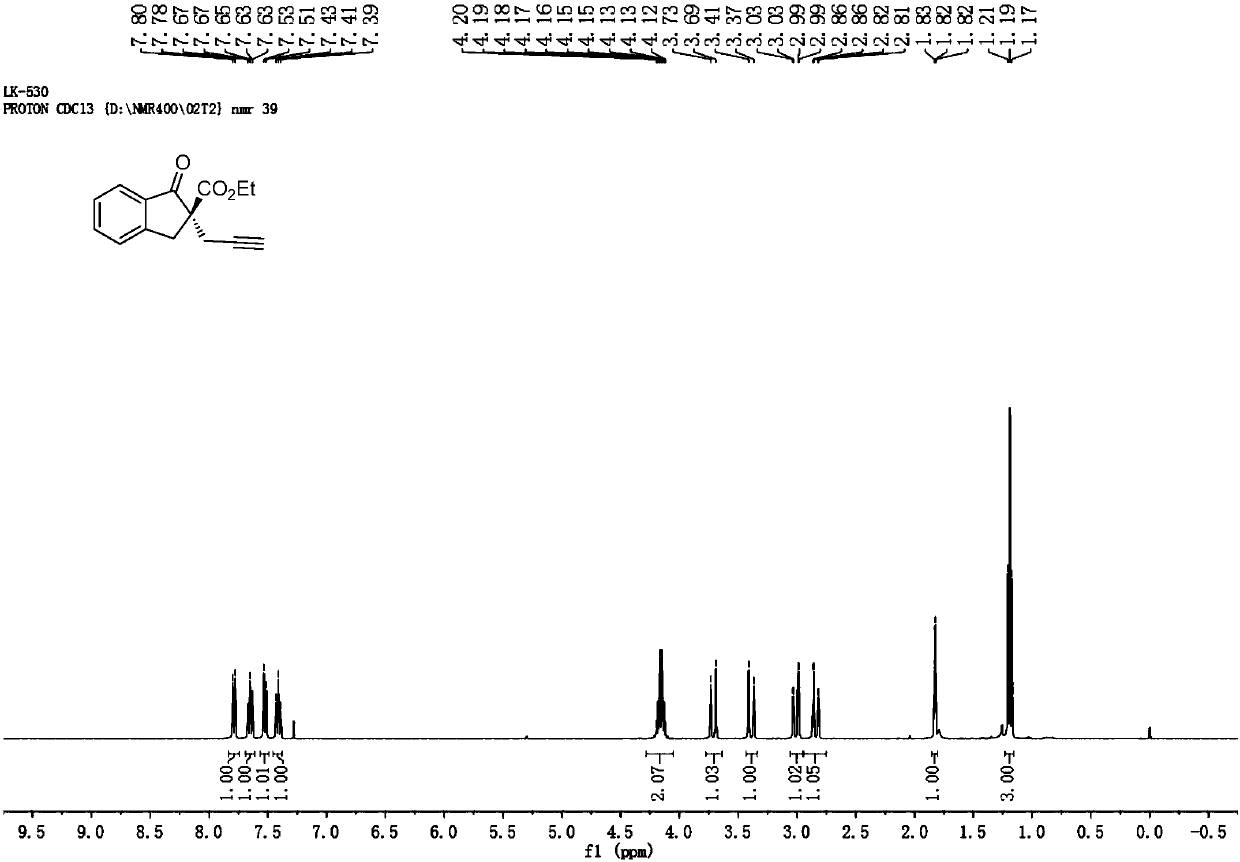
[0066] L-1-1 reacts as a ligand to generate product I-1
[0067] The ligand L-2-1 in Example 1 was replaced with the ligand L-1-1, the temperature was room temperature, and the rest were the same as those in Example 1. The reaction gave compound I-1 in 39% yield, 7% ee.
[0068] The structural formula of L-1-1 is as follows:
[0069]
Embodiment 3
[0071] L-2-2 reacts as a ligand to generate product Ⅰ-1
[0072] The ligand L-2-1 in Example 1 was replaced by the ligand L-2-2, and the temperature was room temperature, and the rest were the same as those in Example 1. The reaction gave compound I-1 in 70% yield, 66% ee.
[0073] The structural formula of L-2-2 is as follows:
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com