N,N,N',N'-dodecyl-tetrasubstituted diphenyl ether sulfonate anionic gemini surfactant and synthesis method thereof
A technology of diphenyl ether sulfonate and dodecyl tetradecyl, which is applied in the direction of surface active detergent composition, sulfonate preparation, sulfonic acid preparation, etc., can solve the problem of gemini surfactant lateness, and achieve easy separation and purification, the synthesis method is simple, and the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] In order to illustrate the present invention more clearly, the following further describes the present invention in combination with preferred embodiments. Those skilled in the art should understand that the content described below is illustrative rather than restrictive, and should not limit the protection scope of the present invention.
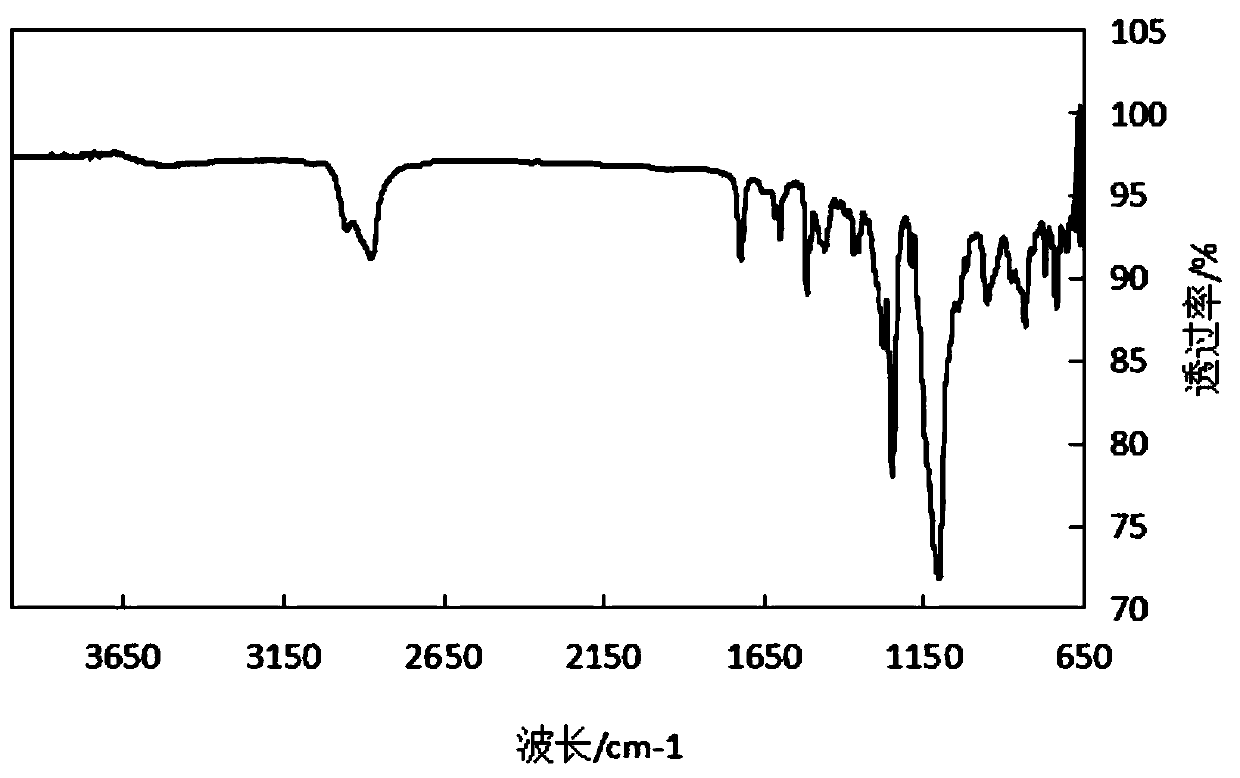
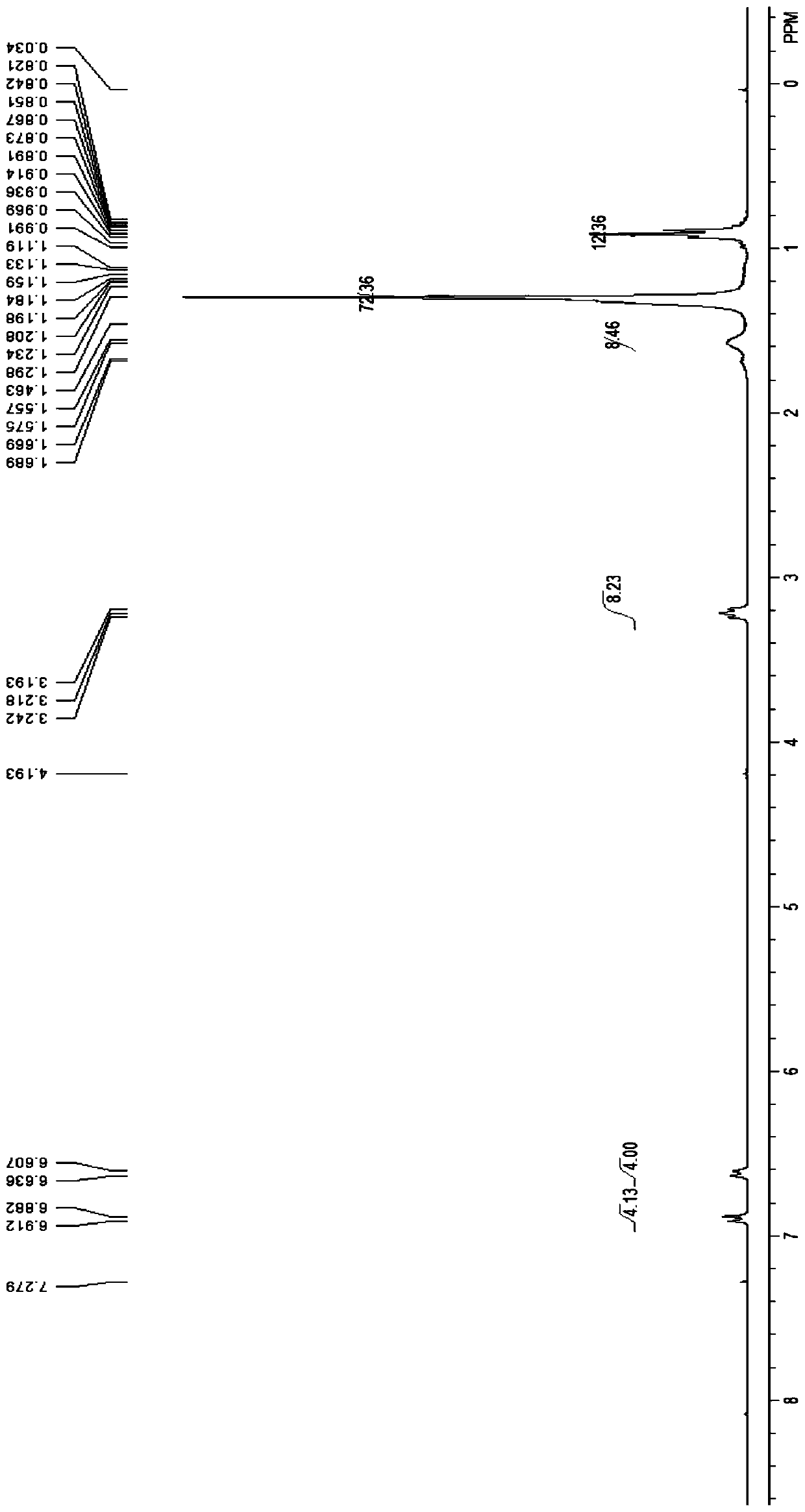
[0041] Preparation of N,N,N′,N′-dodecyl tetrasubstituted diphenyl ether sulfonate
[0042] (1) Synthesis of N,N,N′,N′-dodecyl tetrasubstituted diphenyl ether
[0043] Put 25.00g (124.85mmol) 4,4'-diaminodiphenyl ether, 10.46g (41.95mmol) bromododecane, 5.80g K in a three-necked flask equipped with a stirrer in a constant temperature water bath. 2 CO 3 (20wt%) as an acid binding agent, maintain the pH of the system = 7-10, and add 150 mL of DMF as a solvent under the protection of nitrogen. Stir and raise the temperature to 60°C. After 24 hours of reaction, the reaction is complete (TLC monitors the end of the reaction, the developing solve...
PUM
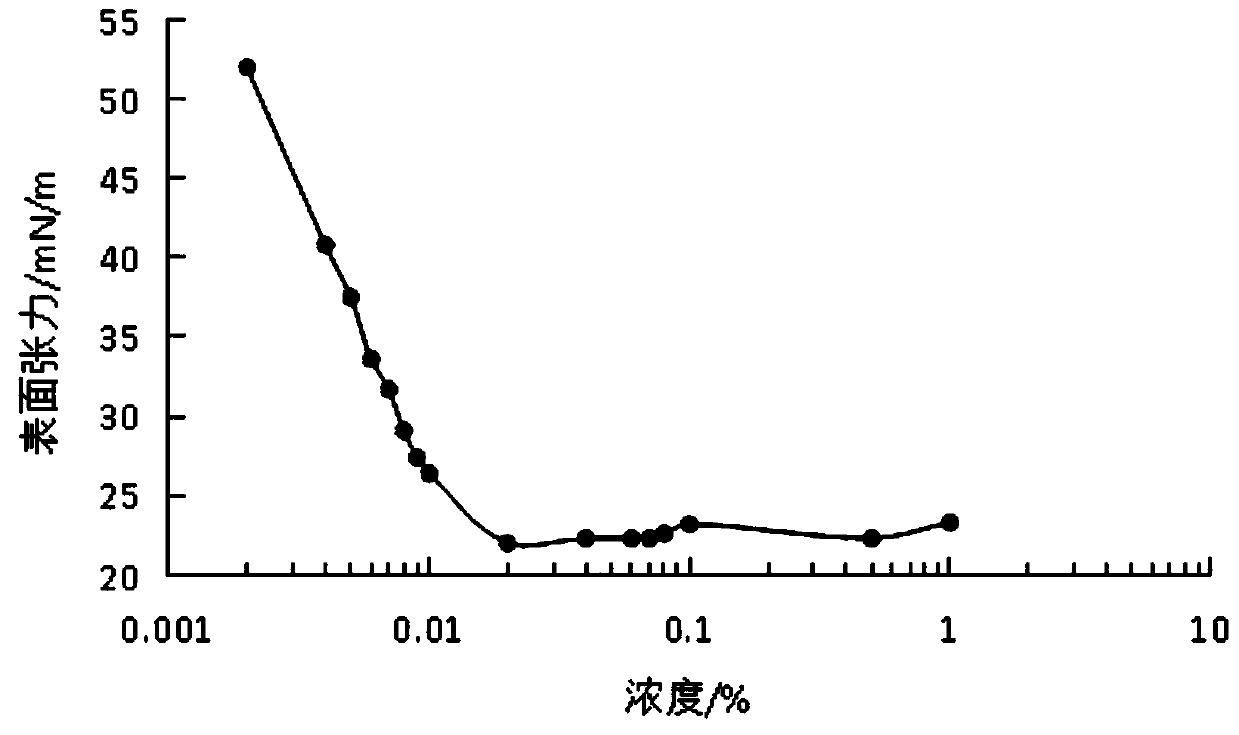
| Property | Measurement | Unit |
|---|---|---|
| surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com