Heterocyclic compound and use thereof
A kind of technology of heterocyclic compound and heteroaryl, applied in the field of organic electroluminescent materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0110] The specific embodiment of the present invention also provides the above-mentioned preparation method, which is synthesized through the following general synthetic route:
[0111]
[0112] The bromides of anthracene derivatives are chemically coupled with organoborides of dibenzofuran or dibenzothiophene derivatives according to the Suzuki-Miyaura reaction using tetrakis(triphenylphosphine)palladium(0) as a catalyst.
[0113] in,
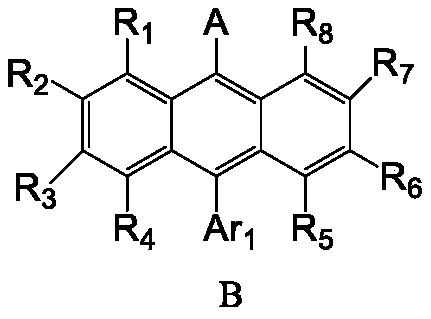
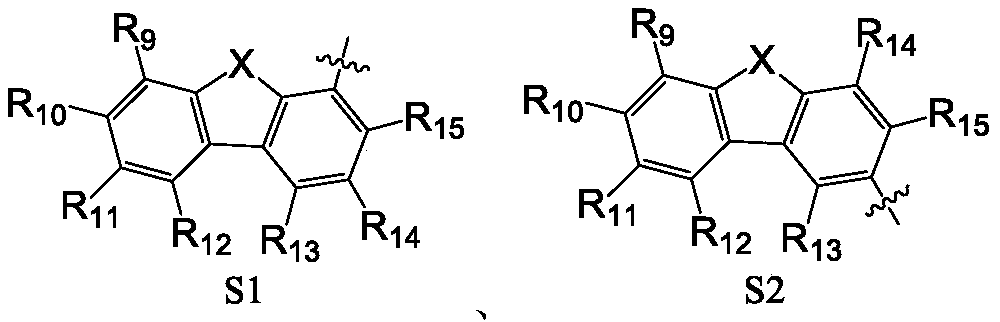
[0114] Ar 1 selected from hydrogen atoms, substituted or unsubstituted C6-C30 aryl groups;
[0115] R 1 -R 8 each independently selected from a hydrogen atom, a deuterium atom, a C1-C8 alkyl group, a substituted or unsubstituted C6-C30 aryl group;
[0116] X is selected from O or S;
[0117] R 9 -R15 Each is independently selected from a hydrogen atom, a deuterium atom, a C1-C8 alkyl group, a substituted or unsubstituted C6-C30 aryl group, a substituted or unsubstituted C6-C30 heteroaryl group.
[0118] Synthetic example:
[0119] ...
Embodiment 1
[0121] The preparation of embodiment 1:L2
[0122]
[0123] After adding a certain amount of D2 and K2 into the three-necked flask, install a mechanical stirring bar, feed nitrogen for 20 minutes, and add catalyst Pd (PPh 3 ) 4 0.25-3mol%, 0.018mol of 2M alkali solution, heated to reflux, reacted for 5-10 hours, suction filtered after reaction, washed with toluene and ethanol. After recrystallization from xylene, a powder with a purity of more than 99% is obtained. In order to further improve the purity of L2, a vacuum sublimation apparatus is used to perform one or more sublimation, and an L2 product with a purity greater than 99.5% can be obtained.
[0124] Using CDCL 3 Used as a solvent, tetramethylsilane (δ = 0.00ppm) as an internal standard record 1 H NMR spectrum.
[0125] 1H NMR (400MHZ, DMSO-d6):
[0126] 7.32ppm(12H,p), 7.38-7.41ppm(4H,t), 7.48ppm(2H,d), 7.54ppm(2H,d), 7.63-7.71ppm(14H,m).
Embodiment 2
[0127] The preparation of embodiment 2:L5
[0128]
[0129] After adding a certain amount of D5 and K5 into the three-necked flask, install a mechanical stirring bar, feed nitrogen for 20 minutes, and add catalyst Pd (PPh 3 ) 4 0.25-3mol%, 0.018mol of 2M alkali solution, heated to reflux, reacted for 5-10 hours, suction filtered after reaction, washed with toluene and ethanol. After recrystallization from xylene, a powder with a purity of more than 99% is obtained. In order to further improve the purity of L5, a vacuum sublimation apparatus is used to carry out one or more sublimation, and the L5 product with a purity greater than 99.5% can be obtained.
[0130] Using CDCL 3 Used as a solvent, tetramethylsilane (δ = 0.00ppm) as an internal standard record 1 H NMR spectrum.
[0131] 1 H NMR (400MHZ, DMSO-d6):
[0132] 7.19-7.22ppm (4H, q), 7.32ppm (12H, t), 7.41-7.48ppm (8H,p), 7.67ppm (8H,p).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com