Micrornas as biomarkers for endometriosis
A technology for endometriosis and heterotopia, which is used in the determination/inspection of microorganisms, biological testing, biological material analysis, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0222] Example 1: Serum microRNAs as diagnostic markers for endometriosis; comprehensive array-based analysis
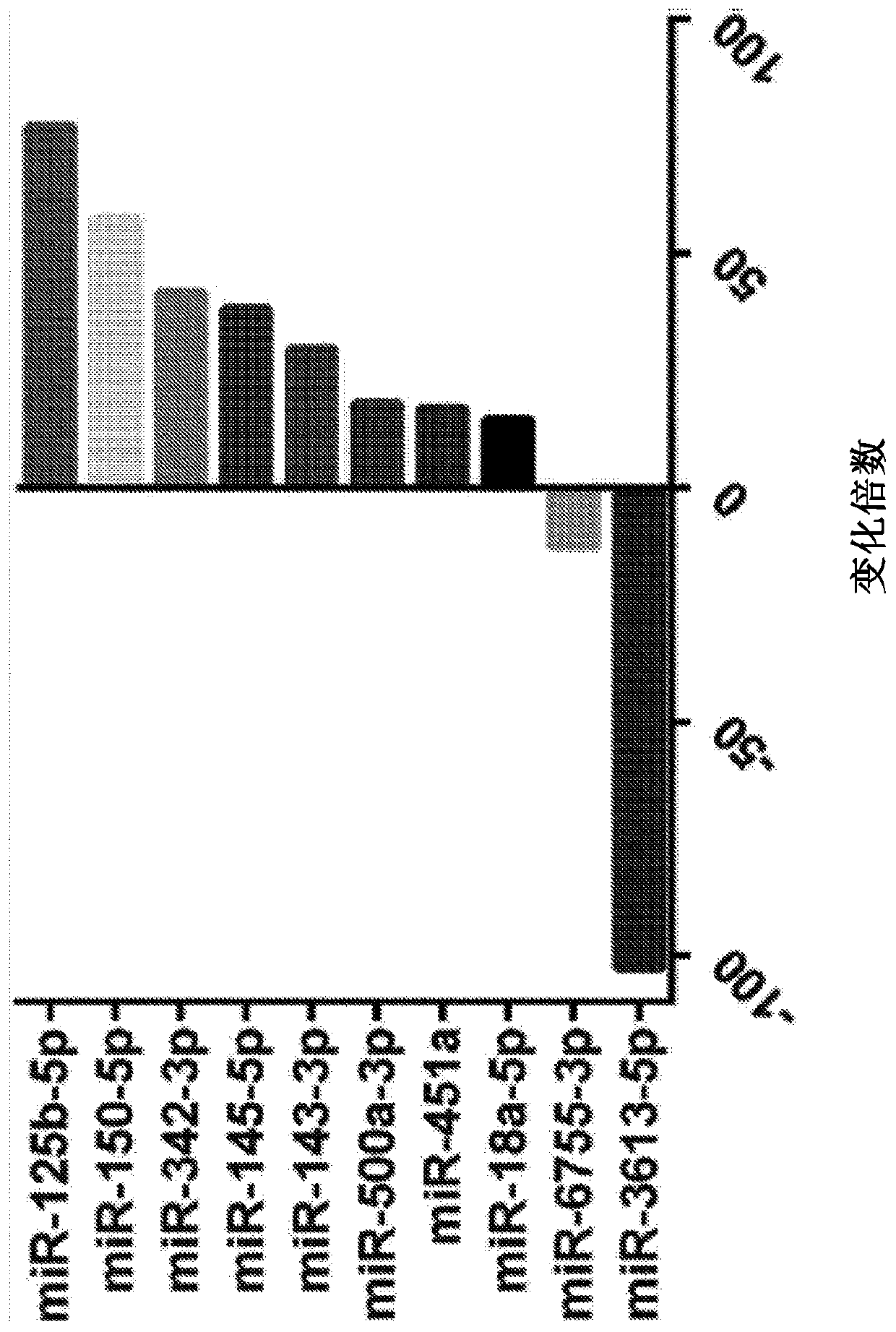
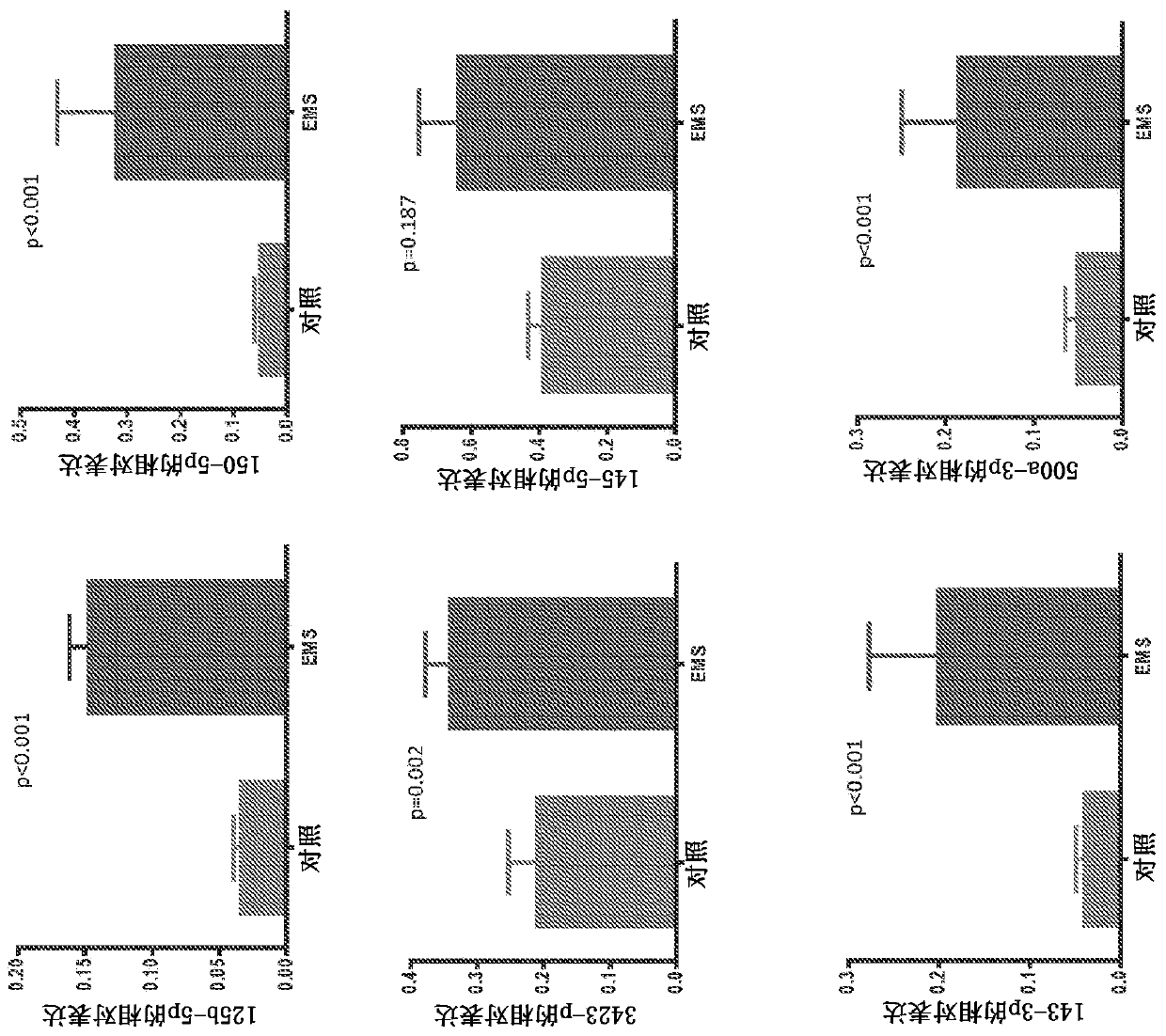
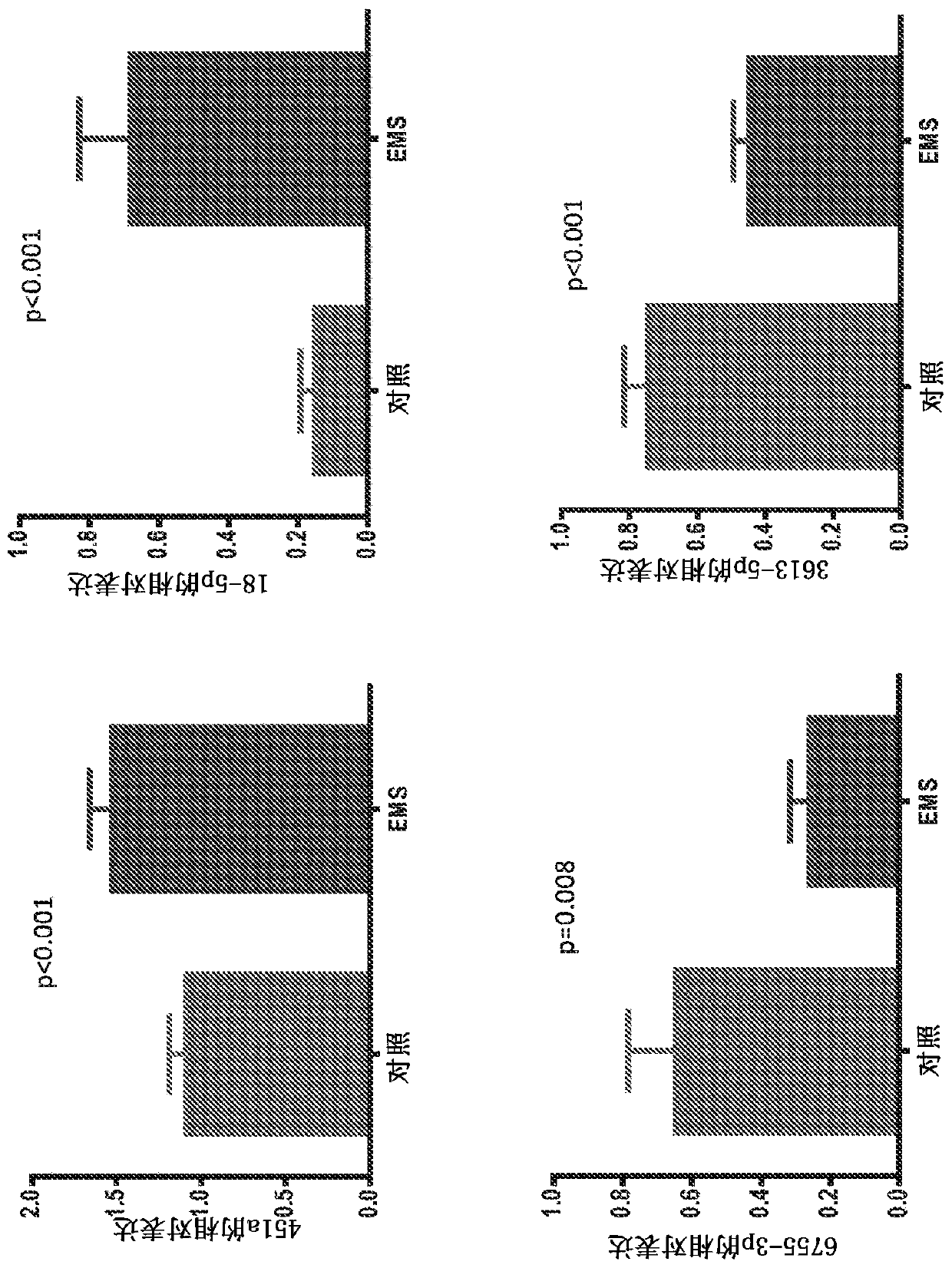
[0223]Microarray studies of ectopic and ectopic endometrial tissue from women with and without endometriosis revealed differential expression of some miRNAs (Ohlsson et al., 2009, Mol Endocrinol, 23:265- 275, Petracco et al., 2011, J Clin Endocrinol Metab, 96:E1925-1933). They play an important role in the pathogenesis of endometriosis and related infertility by regulating gene expression (Teague et al., 2010, HumReprod Update, 16:142-165). Tissue miRNAs are shed from pathological tissues into the circulation, and a strong correlation has been shown between circulation and tissue levels (Resnick et al., 2009, Gynecol Oncol, 112:55-59). Some differentially expressed miRNAs have been identified in the serum of patients with endometriosis (jia et al., 2013, Hum Reprod, 28:322-330, Wang et al., 2013, J Clin Endocrinol Metab, 98: 281 -289). It has previously been sho...
Embodiment 2
[0256] Example 2: Relative expression of miRNAs in endometriotic lesions in baboons
[0257] Such as Figure 4 As shown, the relative expression of miRNAs in endometriotic lesions in baboons was determined by real-time quantitative PCR. The relative expression levels of miR-125b-5p, miR-150-5p and miR-3613-5p were compared to the relative expression levels of the respective miRNAs after statin treatment. Lesion shrinkage was observed in statin treated animals. Expression levels of miR-125b-5p in samples from untreated animals were shown to have higher relative expression levels than samples taken from treated animals. Furthermore, it was observed that the relative level of miR-3613-5p was lower in samples from control untreated animals compared to the level of miR-3613-5p in samples from treated animals. Finally, it was observed that the relative level of miR-150-5p was lower in samples from control untreated animals compared to the level of miR-150-5p in samples from tre...
Embodiment 3
[0258] Example 3: Salivary microRNA as a diagnostic marker for endometriosis
[0259] Step 1: Extraction of RNA from saliva
[0260]Saliva samples (200 μL) were collected and transferred to 1.5 mL tubes. RNase-free water was added to the samples in volumes less than 200 μL to bring the total sample volume to 200 μL. 1 mL of QIAzol Lysis Reagent (Qiagen) was added to the sample. The tubes were vortexed briefly and the samples were incubated at room temperature for five minutes. Then, 200 μL of chloroform was added to the lysate and vortexed for about 15 seconds. The sample mixture was then incubated at room temperature for 2 minutes and centrifuged at 12,000 xg for 15 minutes in a cryogenic chamber (approximately 4°C). Transfer approximately 560 µL of the aqueous phase to a new 1.5 mL tube. Add 840 μL of 100% ethanol to 560 μL of the aqueous phase to obtain a total volume of 1400 μL. Then, transfer 700 μL of the mixture to an RNeasyMiniElute spin column with a 2 mL co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com