Synthesis method of oxadiazon intermediate
A synthesis method and intermediate technology, applied in the field of synthesis of oxadiazone intermediates, can solve the problems of polluting the environment, difficulty in recycling waste acid and waste water, etc., and achieve the effects of avoiding pollution, great application value, and increased yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
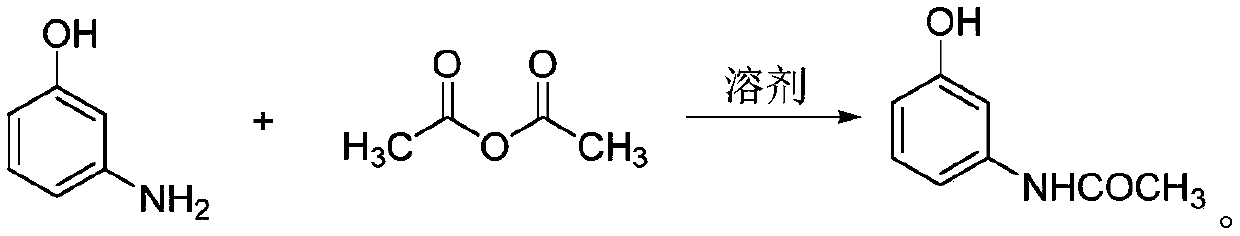
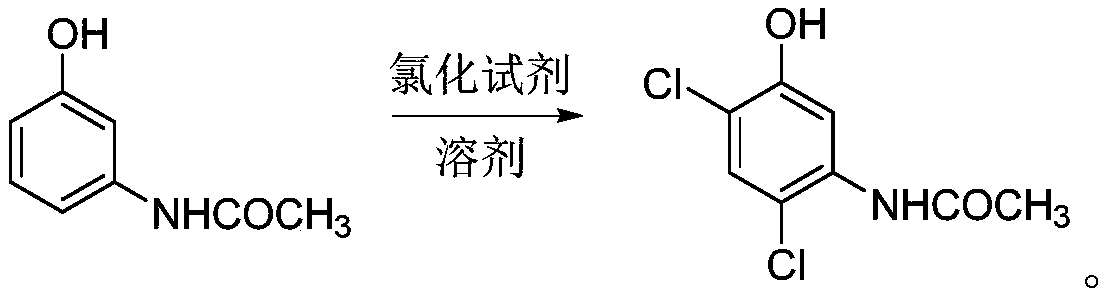
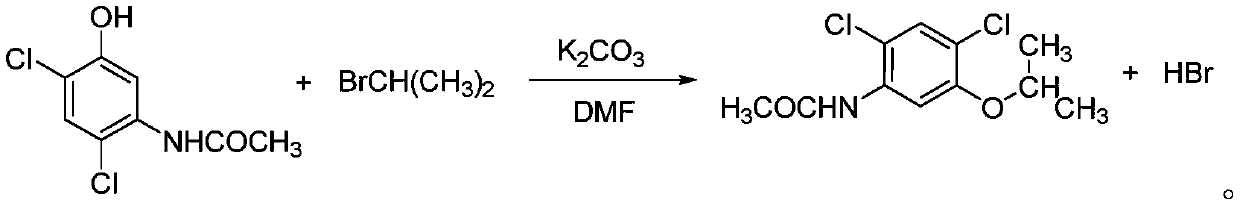
[0045] The invention discloses a synthesis method of an oxadiazone intermediate, specifically discloses a synthesis method of an oxadiazone intermediate amine ether, comprising the following steps:
[0046] S1, take m-aminophenol as the initial raw material;
[0047] S2, performing an acylation reaction on m-aminophenol to obtain a reaction solution A;
[0048] S3, performing a chlorination reaction on the reaction solution A to obtain 2,4-dichloro-5-hydroxyacetanilide;
[0049] S4. Etherifying 2,4-dichloro-5-hydroxyacetanilide to obtain 2,4-dichloro-5-isopropoxyacetanilide;
[0050] S5. Deprotecting 2,4-dichloro-5-isopropoxyacetanilide to obtain 2,4-dichloro-5-isopropoxyacetaniline.
[0051] Wherein, the step of acylation reaction is:
[0052] Add m-aminophenol into the acylation solvent and stir to dissolve;
[0053] Add acetic anhydride dropwise to the stirred solution, and carry out acylation reaction under heating conditions to obtain m-hydroxyaniline solution, which ...
Embodiment 1
[0088] (1) Acylation reaction:
[0089] In the 500ml four-neck round-bottomed flask equipped with mechanical stirring, thermometer and condenser tube, add m-aminophenol 55 grams, water 280 grams, acetic anhydride 62 grams, then stir 5 minutes;
[0090] Heating up to 110°C, and under the condition of 110°C, reflux reaction for 3 hours;
[0091] After the reflux reaction finishes, cool to 0 degree and filter to obtain a filter cake;
[0092] Wash the filter cake with a small amount of ice water, and dry the filter cake in a vacuum dryer to obtain 72 grams of off-white 3-hydroxyacetanilide solid, with a yield of 95.4%;
[0093] The 3-hydroxyacetanilide solid was added to a 1000 ml three-neck flask equipped with a thermometer and mechanically stirred, and 400 g of acetonitrile solvent was added and stirred to dissolve.
[0094] (2) Chlorination reaction:
[0095] Under normal temperature conditions, slowly add 145 grams of sulfuryl chloride solution dropwise to the three-necked...
Embodiment 2
[0111] (1) Acylation reaction:
[0112] In a 1000ml four-necked round-bottomed flask equipped with a mechanical stirrer, a thermometer and a condenser, add 55 grams of m-aminophenol and 450 grams of acetonitrile and stir to dissolve at normal temperature;
[0113] Then slowly add 76.5 grams of acetic anhydride to react at normal temperature for 3 hours;
[0114] The end point of the reaction is monitored until the end point of the reaction is reached, and the acetonitrile system is obtained, and the chlorination reaction is carried out.
[0115] (2) Chlorination reaction:
[0116] Under normal temperature conditions, in the acetonitrile system obtained by the acylation reaction, slowly add 150 grams of sulfuryl chloride solution dropwise, after the dropwise addition is completed, react for 3 hours;
[0117] Liquid phase tracking monitors the reaction end point until the reaction end point is reached;
[0118] Then it was cooled to 0° C., the solid was precipitated, and then...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com