Replication-defective recombinant lentivirus CAR-T transgenic vector targeting CD152 and construction method of replication-defective recombinant lentivirus CAR-T transgenic vector
A technology of recombinant lentivirus and transgenic vector, which is applied in the field of medical biology, can solve problems such as slow sales growth, no response, and large side effects, and achieve the effects of reducing endotoxin content, avoiding tediousness and mistakes, and improving safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
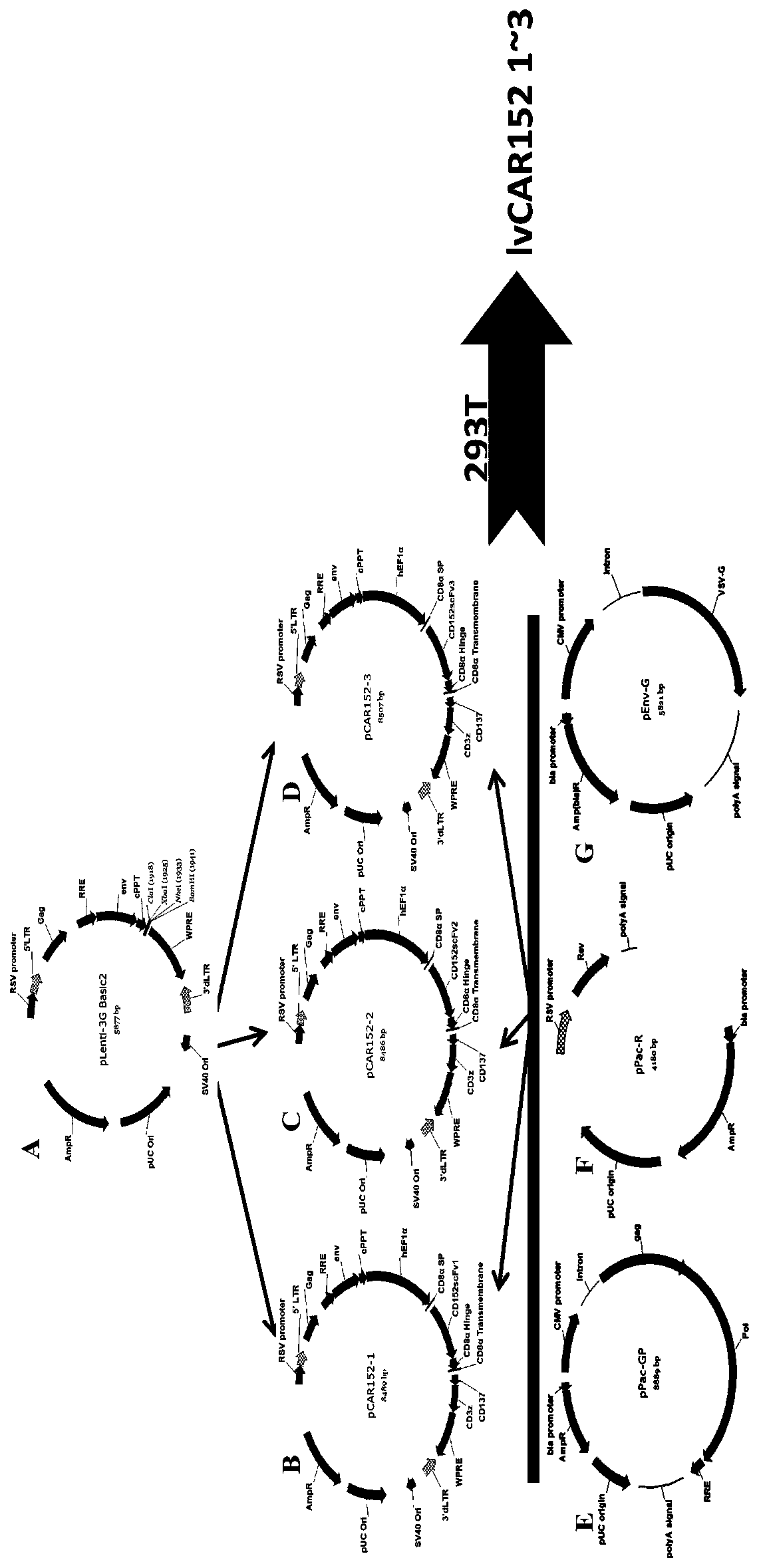
[0110] The construction methods of recombinant lentiviral plasmids pCAR152-0, pCAR152-1, pCAR152-2 and pvCAR152-3 are as follows:
[0111] The promoters and sequences of CAR152-0, CAR152-1, CAR152-2, CAR152-3 (see Table 1) and human EF1α were all synthesized by Gene Synthesis Company. Then, as shown in Table 2, the synthetic human EF1α promoter and CAR structure (CAR152-0, CAR152-1, CAR152-2, CAR152-3) were cloned into the recombinant lentiviral backbone plasmid pLenti-3G basic2 (see attached figure 1 In A), the recombinant lentiviral plasmids pCAR152-0~pCAR152-3 were respectively obtained (pCAR152-0 is a negative control, so the structural diagram is not shown; the structural diagram of pCAR152-1~pCAR152-3 is shown in figure 1 B. figure 1 C and figure 1 D).
[0112] Table 1 Chimeric Antigen Receptor Structure
[0113]
[0114] Table 2 Composition of recombinant lentiviral plasmids
[0115] recombinant lentiviral plasmid Recombinant Lentiviral Backbone Pl...
Embodiment 2
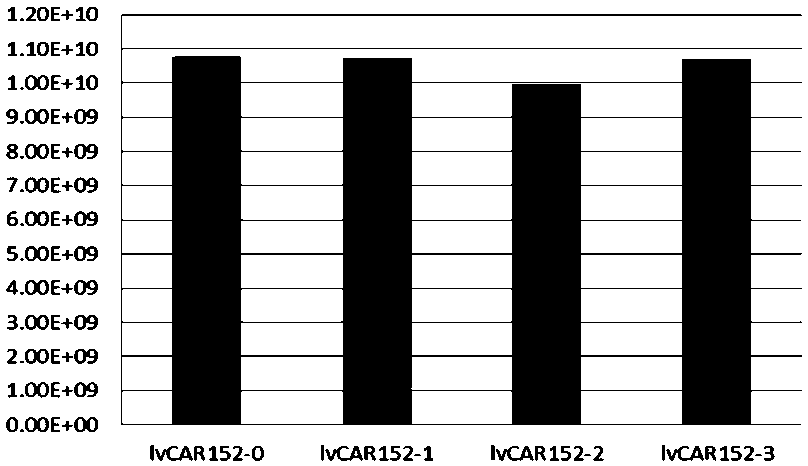
[0132] Obtaining supernatant of lvCAR152-0~lvCAR152-3 recombinant lentiviral vector
[0133]The recombinant lentiviral plasmids pCAR152-0~pCAR152-3 obtained in Example 1 (see the structure diagram of pCAR152-1 figure 1 B. Schematic diagram of pCAR152-2 structure, see figure 1 C. The structure diagram of pCAR152-3 is shown in figure 1 C; because pCAR152-0 is a negative control, so it is not shown in the figure) and the packaging plasmid (see the schematic diagram of the structure) respectively figure 1 E. figure 1 F. figure 1 G) co-transfect into 293T cells, and collect the cell supernatant containing the recombinant lentiviral vectors lvCAR152-0-lvCAR152-3 after 72 hours of transfection.
[0134] 2.1. Complete medium: Take out the preheated fresh medium, add 10% FBS + 5ml Pen-Srep, and mix upside down;
[0135] 2.2. 1XPBS solution: Weigh NaCl 8g, KCl 0.2, NaCl 2 HPO 4 .12H 2 O 3.58g, KH 2 PO4 0.24g
[0136] 2.3. Put it in a 1000ml beaker, add 900ml Milli-Q grade ultr...
Embodiment 3
[0157] Purification of recombinant lentiviral vector lvCAR152-0~lvCAR152-3
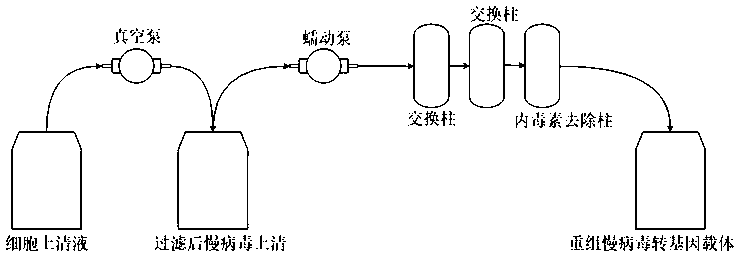
[0158] Purification flow chart such as figure 2 As shown, firstly, the cell supernatant collected in 2.21 is filtered by a vacuum pump, and then the impurities and endotoxins are removed through the ion exchange column and the endotoxin removal column, and then injected into the ion exchange column at a certain flow rate by the peristaltic pump. , and eluted to obtain a harvested solution of replication-deficient lentiviral vectors. The lvCAR152 eluted by this method is more pure and easier to operate.
[0159] The specific operation steps are as follows:
[0160] 3.1. Use a Thermo vacuum pump to filter the collected supernatant through a 0.22μm-0.8μm PES filter to remove impurities;
[0161] 3.2. Add 1.5M NaCl 250mM Tris-HCl (pH 6-8) to the supernatant in an appropriate proportion;
[0162] 3.3. Place two ion-exchange columns and endotoxin removal columns in series, and pass through the columns ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com