Brivaracetam isomer detection method
A detection method and technology for isomers, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
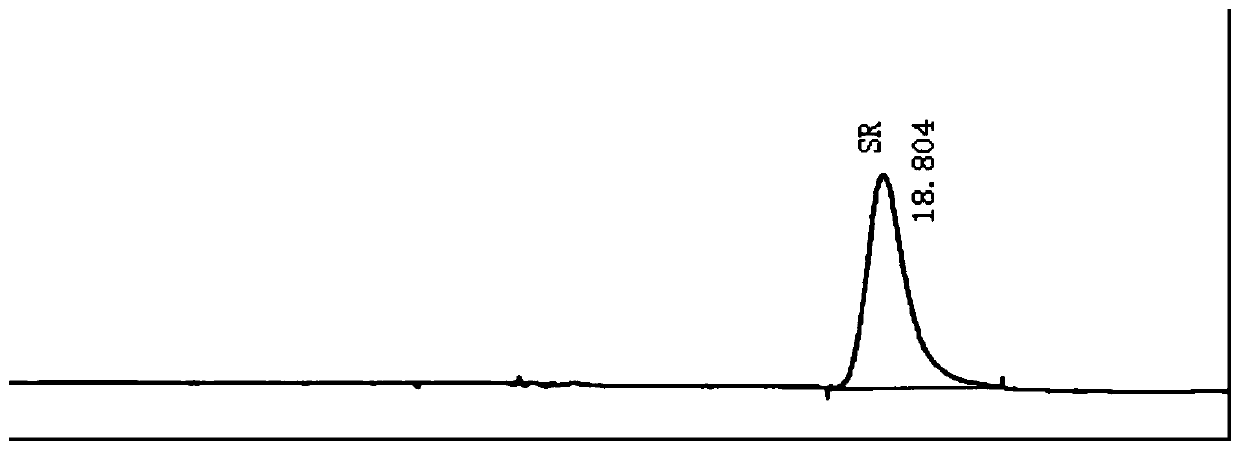
[0052] Embodiment 1 high-performance liquid chromatography is measured Bu Waracetam enantiomer content
[0053] 1. Main technical parameters of high performance liquid chromatography
[0054] a. High performance liquid chromatography;
[0055] b. A chiral chromatographic column with amylose-tris(3,5-xylylcarbamate) as the stationary phase covalently bonded to the surface of silica gel;
[0056] c, mobile phase: normal hexane: ethanol: trifluoroacetic acid=85:15:0.1, flow velocity is 1.0ml / min;
[0057] d. The detection wavelength is 210nm, and the column temperature is 30°C.
[0058] 2. Detection
[0059] (1) Prepare the solution
[0060]a. The test solution: Accurately weigh 49.45mg of brivaracetam sample (batch number 20180501, provided by Chengdu Weibang Pharmaceutical Co., Ltd.), place it in a 50ml volumetric flask, dilute to the mark with the mobile phase, and use it as the test solution .
[0061] b. Control solution: Accurately measure 1.0ml of the test solution i...
Embodiment 2
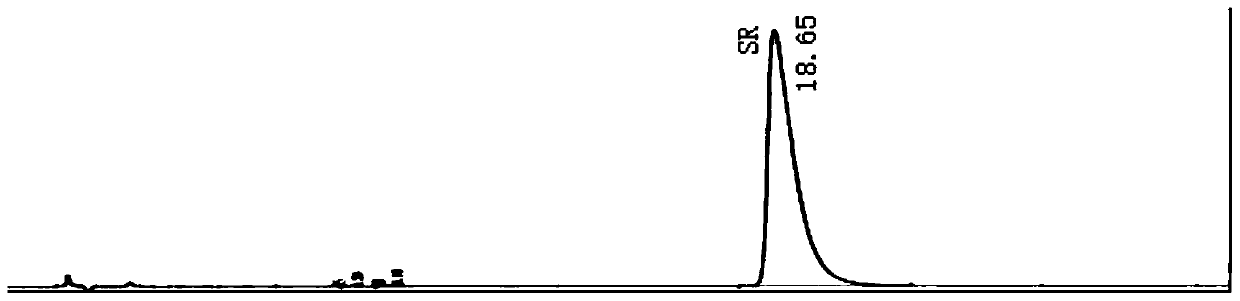
[0085] Embodiment 2 high-performance liquid chromatography is measured Bu Waracetam enantiomer content
[0086] 1. Main technical parameters of high performance liquid chromatography
[0087] a. High performance liquid chromatography;
[0088] b. A chiral chromatographic column with amylose-tris(3,5-xylylcarbamate) as the stationary phase covalently bonded to the surface of silica gel;
[0089] c, mobile phase: normal hexane: ethanol: trifluoroacetic acid=85:15:0.1, flow velocity is 1.0ml / min;
[0090] d. The detection wavelength is 210nm, and the column temperature is 30°C.
[0091] 2. Detection
[0092] (1) Prepare the solution
[0093] a. The test solution: Accurately weigh 49.54mg of brivaracetam sample (batch number 20180801, provided by Chengdu Weibang Pharmaceutical Co., Ltd.), place it in a 50ml volumetric flask, dilute to the mark with the mobile phase, and use it as the test solution .
[0094] b. Control solution: Accurately measure 1.0ml of the test solution ...
experiment example 1
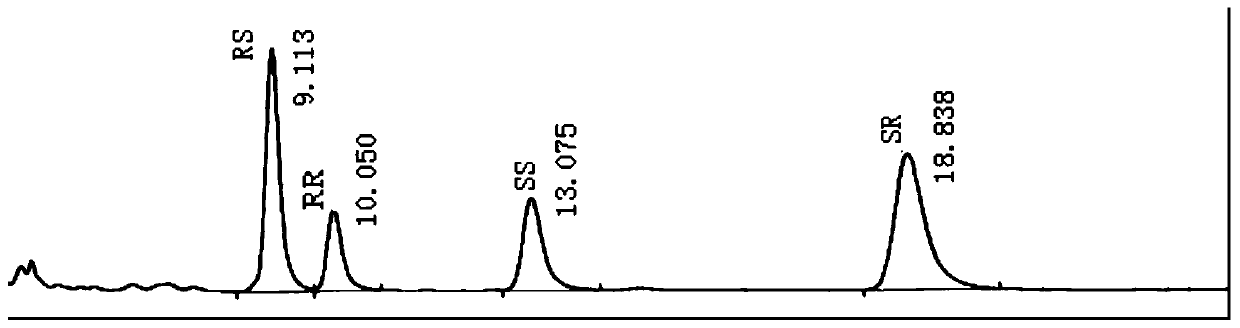
[0119] Experimental Example 1 System Applicability Investigation
[0120] Solution preparation: Accurately weigh the appropriate amount of Buvaracetam reference substance (batch number 20171201) and Brivaracetam isomer reference substance, and prepare it with mobile phase (the volume ratio of n-hexane:ethanol:trifluoroacetic acid is 85:15:0.1) Prepare a system suitability solution with a concentration of brivaracetam of 1.0 mg / ml and each isomer of 0.01 mg / ml. Take 20 μl of the test solution, inject it into the liquid chromatograph, inject the sample 6 times, investigate the relative standard deviation of retention time and peak area, the results are shown in Table 7.
[0121] Table 7 Summary of system suitability solution test results
[0122]
[0123] Conclusion: the test solution was injected continuously for 6 times, and the RSD of the peak area of brivaracetam was 0.6%, not more than 2%, which met the requirements; the RSDs of each isomer were 1.95%, 2.73%, and 2.34...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com