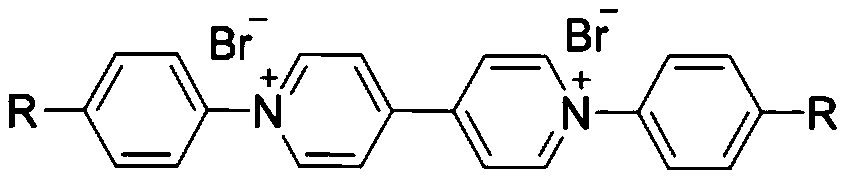
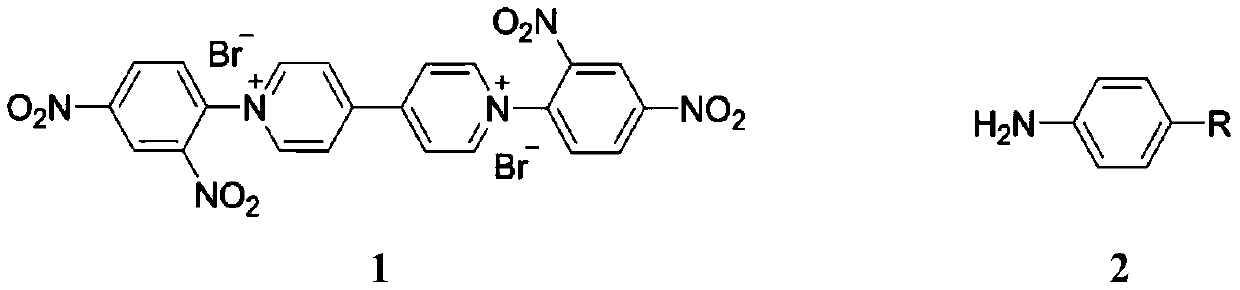
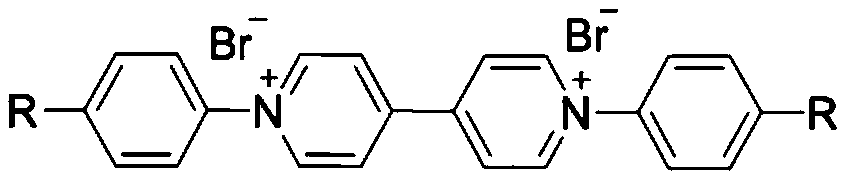
First-class purpurin ion monomers and preparation method and application thereof
A technology of ionic monomer and viologen, which is applied in the field of viologen ionic monomer and its preparation, can solve the problems of insufficient rigidity, difficulty in synthesizing a porous framework structure with high specific surface area, etc., and achieve short reaction time, rich variety and excellent reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the preparation of viologen base Zincke salt VL-Br
[0025]
[0026] Weigh 4,4'-bipyridine (10mmol, 1.56g) and 1-bromo-2,4-dinitrobenzene (20mmol, 4.94g) in a reaction flask, add 100mL solvent acetonitrile, stir to make the raw materials completely dissolve. Then, the above solution was refluxed and stirred at 90° C. for 72 h. After the reaction, the resulting solid product was simply filtered, washed and dried to obtain a yellow solid, viologenyl Zincke salt VL-Br, with a yield of 90%. Characterized as follows:
[0027] 1 13C NMR( 100MHz, D2O): δ152.58, 149.81, 146.80, 142.77, 138.19, 131.11, 130.70, 127.54 and 122.77ppm. Elemental analysis: measured value: C, 40.85; H, 2.51; N, 12.83wt%. Theoretical value C22W14O8N9Br62(M. ): C, 40.64; H, 2.17; N, 12.93wt%.
Embodiment 2
[0028] Example 2: Preparation of aniline functionalized viologen ionic monomer
[0029]
[0030] Preparation of viologen ionic monomers functionalized with aniline groups: First, weigh Zincke salt VL-Br (10mmol, 6.50g) and p-phenylenediamine (20mmol, 2.16g) in a reaction flask, add 200mL solvent ethanol , stirred to disperse the raw materials evenly, and heated to reflux at 80°C for 48h. After the reaction, add 50 mL of water and stir for several hours, filter with a common funnel, the filtrate is dark brown, add 10 g of activated carbon and stir for 2 hours after the filtration, and then filter, the color of the filtrate becomes lighter. The filtrate was rotary evaporated under low pressure to remove water to obtain a dark brown solid, which was washed several times with tetrahydrofuran (THF). Finally dried in a vacuum oven to obtain dark brown solid product V-NH 2 -Br.
Embodiment 3
[0031] Embodiment 3: Preparation of benzonitrile functionalized viologen ionic monomer
[0032]
[0033] Preparation of benzonitrile-functionalized viologen ion monomer: First, weigh Zincke salt VL-Br (10mmol, 6.50g) and p-aminobenzonitrile (20mmol, 2.36g) in a reaction flask, add 200mL Solvent ethanol, stir to disperse the raw materials evenly, and heat to reflux at 80°C for 48h. After the reaction, add 50 mL of water and stir for several hours, filter with a common funnel, the filtrate is light brown, and the filtrate is rotated to remove water under low pressure to obtain a light brown solid, which is then washed several times with tetrahydrofuran (THF). Finally, it was dried in a vacuum oven to obtain a light brown solid product V-CN-Br.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com