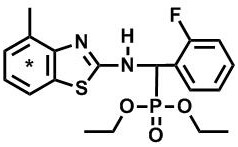
A kind of synthesis method of radioactive isotope carbon-14 labeling poison fluorophos
A technology of radioisotopes and synthesis methods, applied in the field of radiochemical synthesis, can solve the problems of lack of in-depth research on the metabolic process of toxic fluorine and phosphorus and environmental behavior, and achieve the effects of firm labeling, high chemical purity, and not easy to fall off.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
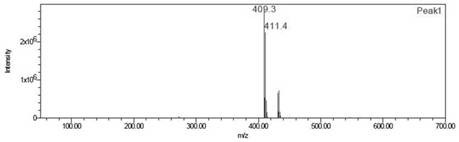
Image
Examples
Embodiment Construction
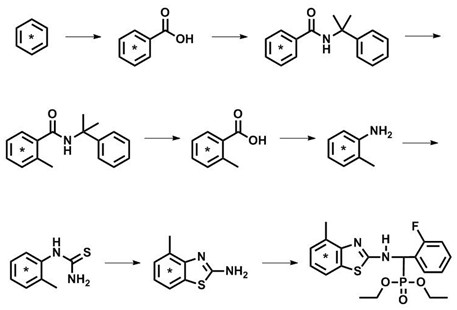
[0024] The following examples are given to illustrate the present invention. The implementation examples are only used to further illustrate the present invention, and do not represent the protection scope of the present invention. Non-essential modifications and adjustments made by others according to the present invention still belong to the protection scope of the present invention.
[0025] At room temperature under a CO atmosphere, trifluoroacetic acid (10 mL) was added to a mixture containing potassium persulfate (1650 mg) and palladium acetate (261 mg), stirred, and then [U- 14 C 6 ] Benzene (65.6 mCi, 25.5 mCi / mmol). Vigorous stirring was continued at atmospheric pressure for 10 h. After the reaction is completed, concentrate the reaction solution to dryness, add water, adjust the pH to 13 with NaOH aqueous solution, extract the aqueous phase with methyl tert-butyl ether to remove impurities, adjust the pH to 1 with concentrated hydrochloric acid, extract with ethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com