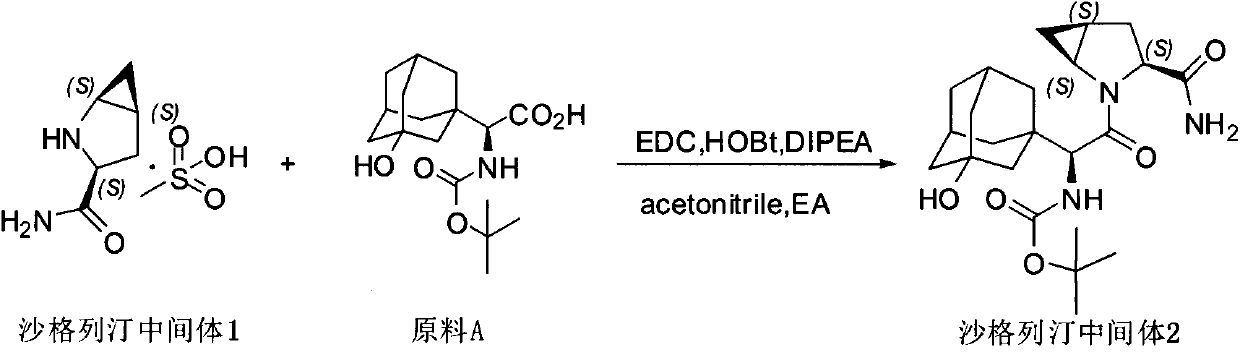
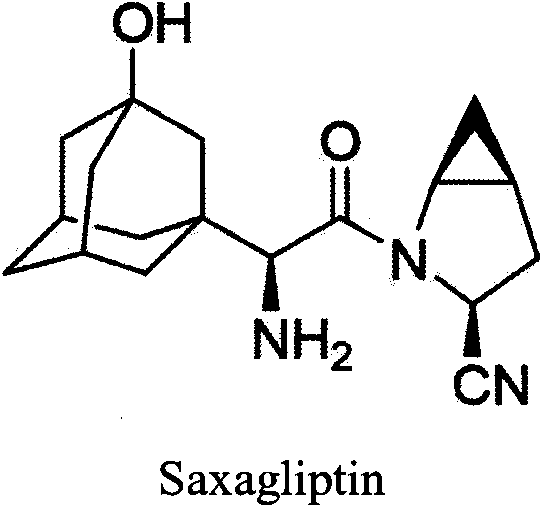
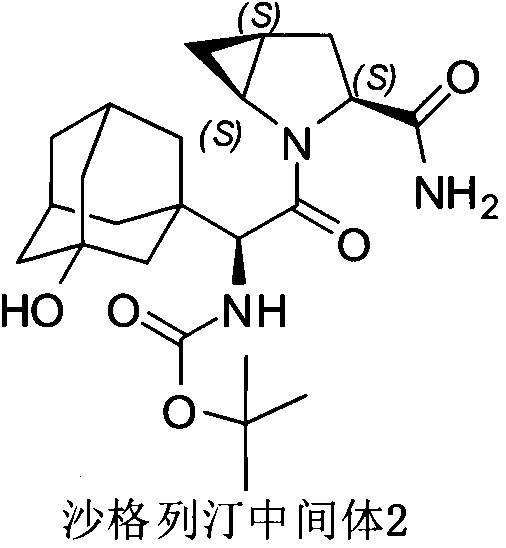
Method for preparing saxagliptin intermediate
An intermediate, acetonitrile technology, applied in the field of preparation of saxagliptin intermediates, can solve problems such as low yield, unfavorable large-scale production, environmental protection, and complicated treatment, so as to improve product yield and purity, and yield is stable , the effect of high-purity post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] Add 2.5kg of acetonitrile, 1.77kg of ethyl acetate, and 2.03kg of N,N-diisopropylethylamine to the reaction kettle in sequence, and stir for later use. Then add raw material A 2.33kg, saxagliptin intermediate 1 1.67kg, hydroxybenzotriazole 1.11kg, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 1.51kg kg, acetonitrile 2.83kg, stirred for 2-3 hours.
[0029] After most of the acetonitrile was distilled off under reduced pressure, 20.90 kg of ethyl acetate was added to the reaction liquid and stirred. After adjusting the pH to neutral, 11.80kg of sodium chloride solution was added, stirred at room temperature, left to stand, and then the water layer was removed. Add 12.76 kg of 20% potassium bicarbonate solution to the organic phase, stir, let stand and remove the water layer. Add 12.76 kg of 20% potassium bicarbonate solution to the organic phase, stir, and remove the water layer after standing.
[0030] Precipitate the organic phase to 4.0-5.9...
Embodiment 2
[0032]
[0033] 10kg of acetonitrile, 7.1kg of ethyl acetate, and 8.1kg of N,N-diisopropylethylamine were successively added into the reaction kettle, and stirred for later use. Then add raw material A 9.32kg, saxagliptin intermediate 1 6.68kg, hydroxybenzotriazole 4.4kg, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 6.0kg kg, acetonitrile 11.3kg, stirred for 3 hours.
[0034] After most of the acetonitrile was distilled off under reduced pressure, 83.6 kg of ethyl acetate was added to the reaction solution and stirred. After adjusting the pH to neutral, add 47.2kg of sodium chloride solution, stir at room temperature, and remove the water layer after standing still. Add 51.0 kg of 20% potassium bicarbonate solution to the organic phase, stir, let stand, and remove the water layer.
[0035] Precipitate the organic phase to 16-25L under reduced pressure, add 23.2kg of ethyl acetate, and then precipitate under reduced pressure to 16-25L; stop the precipitation...
Embodiment 3
[0037]
[0038] 25.0 g of acetonitrile, 17.7 g of ethyl acetate, and 20.3 g of N,N-diisopropylethylamine were successively added into the reactor, and stirred for use. Then add raw material A 23.3g, saxagliptin intermediate 1 16.8g, hydroxybenzotriazole 11.12g, 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride 15.1g g, 28.3 g of acetonitrile, stirred for 2 hours.
[0039] To the reaction liquid, 209.0 g of ethyl acetate was added and stirred. After adjusting the pH to neutral, 118.0 g of sodium chloride solution was added, stirred at room temperature, left to stand, and then the water layer was removed. Add 127.6 g of 20% potassium bicarbonate solution to the organic phase, stir, and remove the water layer after standing. Add 127.6 g of 20% potassium bicarbonate solution to the organic phase, stir, leave to stand and remove the water layer.
[0040] Precipitate the organic phase to 40-60mL under reduced pressure, add 60.0g of ethyl acetate, and then precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com