Liquid phase spherical carrier, and preparation method and applications thereof
A spherical carrier and a carrier technology, which are applied in the field of liquid-phase spherical carriers and their preparation, can solve the problems of increased steric hindrance of polypeptides, inability to effectively monitor the surface substituents of solid-phase synthetic carriers, affecting reaction efficiency, etc., and achieve low steric hindrance. , the effect of facilitating industrial production and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
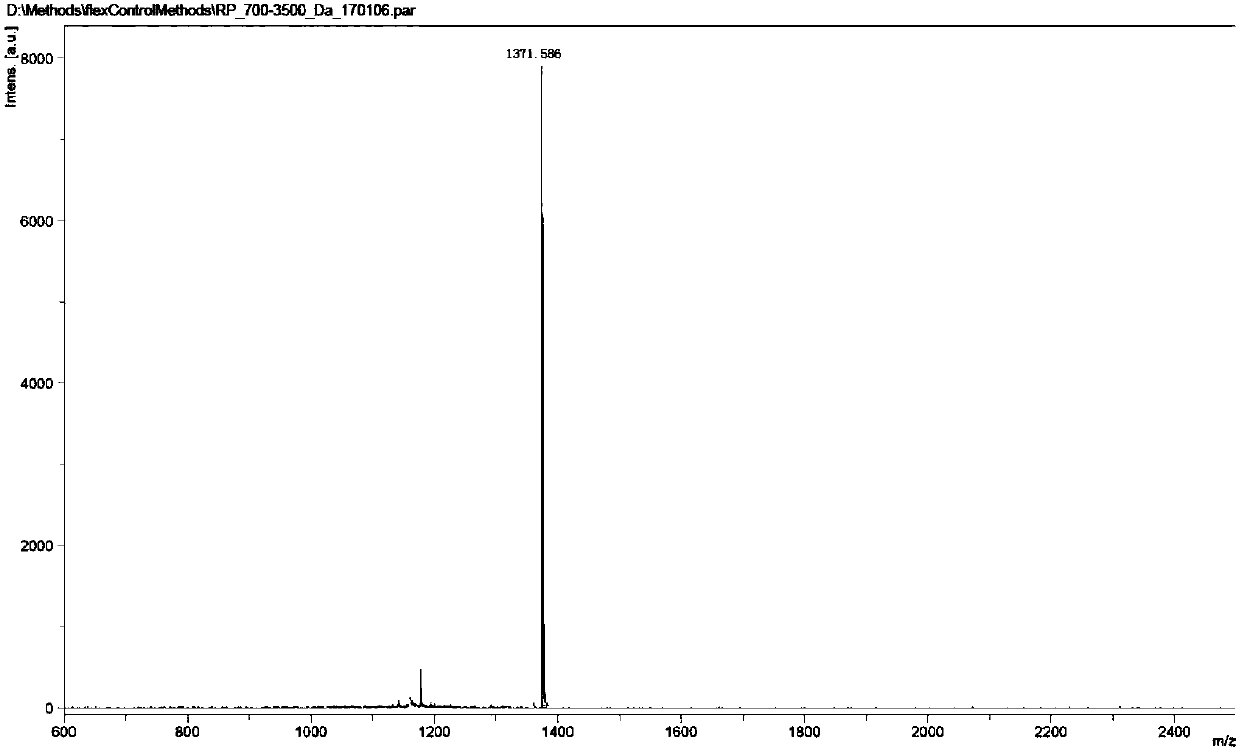
[0047] The synthesis of embodiment 1 compound A
[0048]
[0049] Take compound 1 (C 60 Cl 6 ) 5.6 grams (6mmol) and p-hydroxymethylphenol 7.4 grams (60mmol) were added in the 500mL there-necked flask, then in the reaction flask, DMF (100mL) was added, stirred evenly, then 8.3 grams of potassium carbonate (60mmol) was added. The temperature of the reaction solution was raised to 80° C. and stirring was continued for 16 hours. The reaction was monitored by HPLC. After the raw materials were consumed, the reaction solution was cooled in an ice bath to below 10°C. Under the condition of sufficient stirring, 1 mol / L dilute hydrochloric acid (100 mL) and purified water (200 mL) were slowly added dropwise to the reaction liquid, and stirring was continued for half an hour after the dropwise addition was completed. After the solid precipitated, it was filtered, and the filter cake was washed with purified water (100 mL) and diethyl ether (100 mL) successively. Vacuum drying w...
Embodiment 2
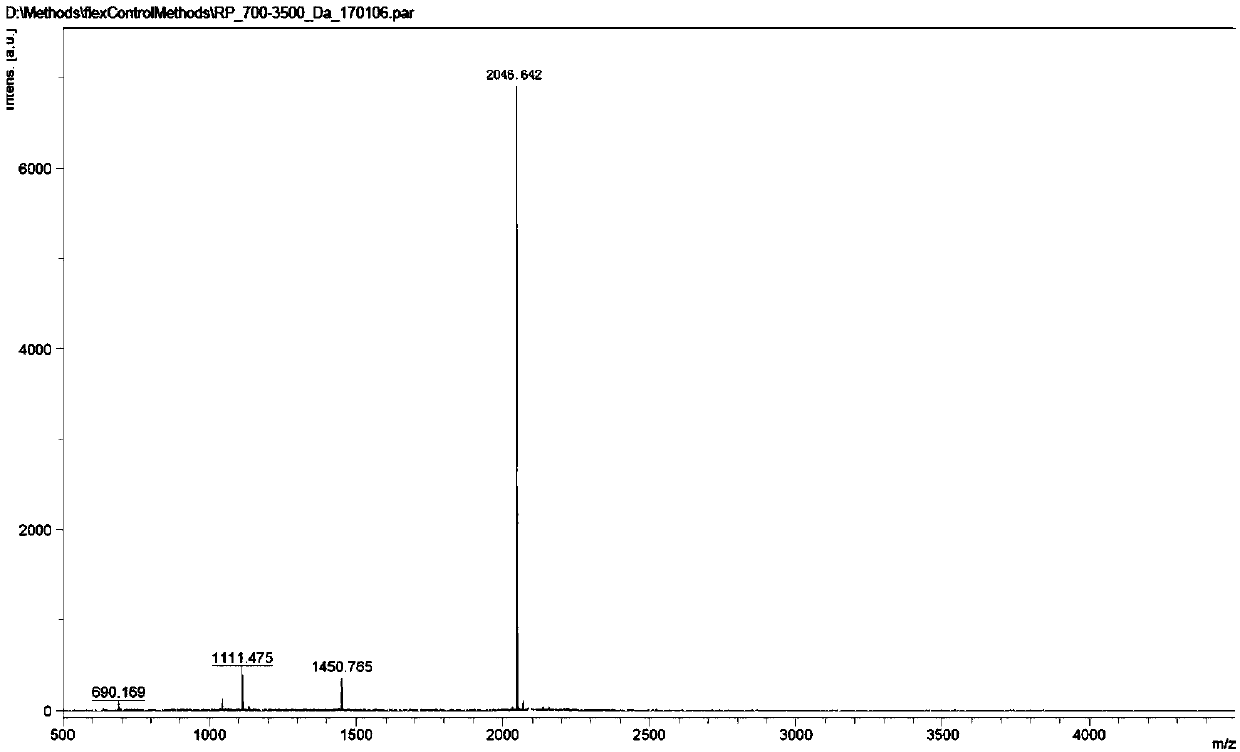
[0050] The synthesis of embodiment 2 compound B
[0051]
[0052] Take 4.7 g (5 mmol) of compound 1 and 12.9 g (50 mmol) of compound 2 into a 1L three-necked flask, then add DMF (150 mL) into the reaction flask, stir well, and then add 6.9 g (50 mmol) of potassium carbonate. The temperature of the reaction solution was raised to 80° C. and stirring was continued for 16 hours. The reaction was monitored by HPLC. After the raw materials were consumed, the reaction solution was cooled in an ice bath to below 10°C. Under the condition of sufficient stirring, 1 mol / L dilute hydrochloric acid (100 mL) and purified water (150 mL) were slowly added dropwise to the reaction liquid, and stirring was continued for half an hour after the dropwise addition was completed. After filtering, the filter cake was washed with purified water (100 mL) and diethyl ether (100 mL) successively. Vacuum drying at 60° C. for 5 hours gave 8.8 g of light yellow solid compound 3 with a yield of 90%. ...
Embodiment 3
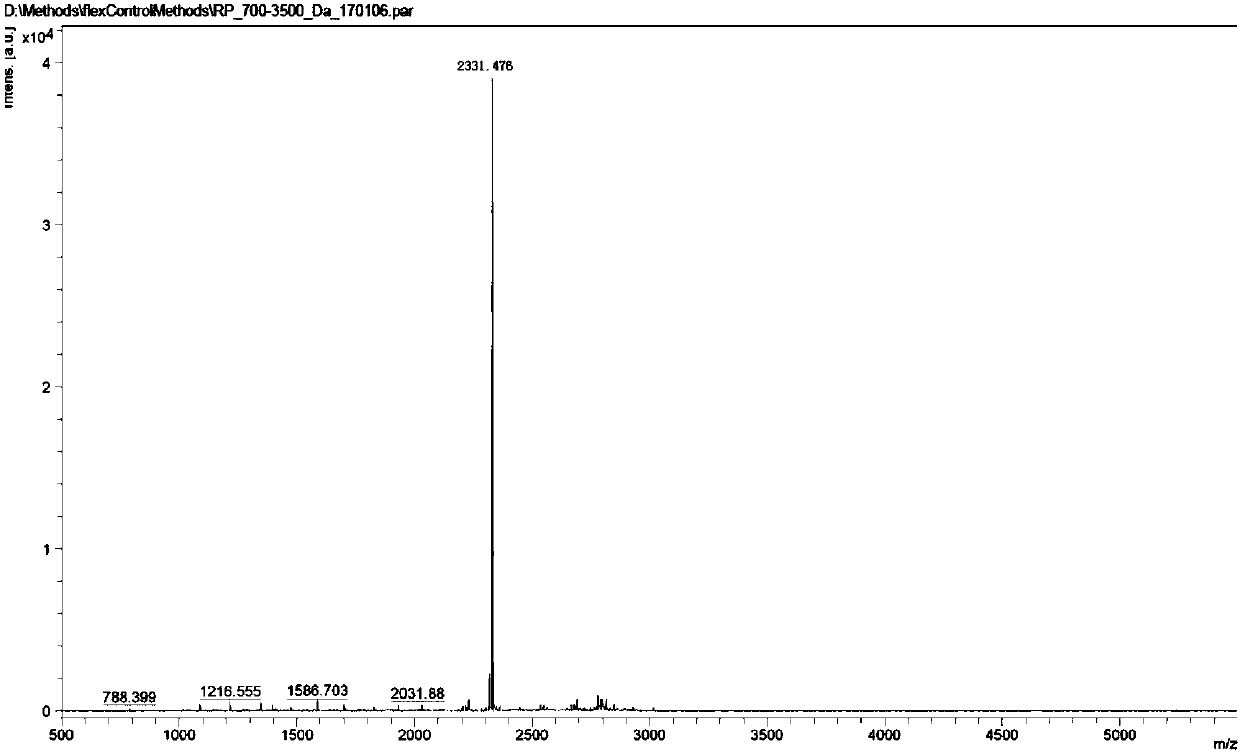
[0056] The synthesis of embodiment 3 compound C
[0057]
[0058] Take compound 1 6.6 grams (7mmol) and Fmoc-NH 2 Add 16.7 grams (70 mmol) into a 1L three-neck flask, then add DMF (150 mL) into the reaction flask, stir evenly, and then add 6.9 grams (50 mmol) of potassium carbonate. The temperature of the reaction solution was raised to 80° C. and stirring was continued for 16 hours. The reaction was monitored by HPLC. After the raw materials were consumed, the reaction solution was cooled in an ice bath to below 10°C. Under the condition of sufficient stirring, 1 mol / L dilute hydrochloric acid (100 mL) and purified water (150 mL) were slowly added dropwise to the reaction liquid, and stirring was continued for half an hour after the dropwise addition was completed. After filtering, the filter cake was washed with purified water (100 mL) and diethyl ether (100 mL) successively. Vacuum drying at 60° C. for 5 hours gave 12.5 g of brown solid compound 6 with a yield of 92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com