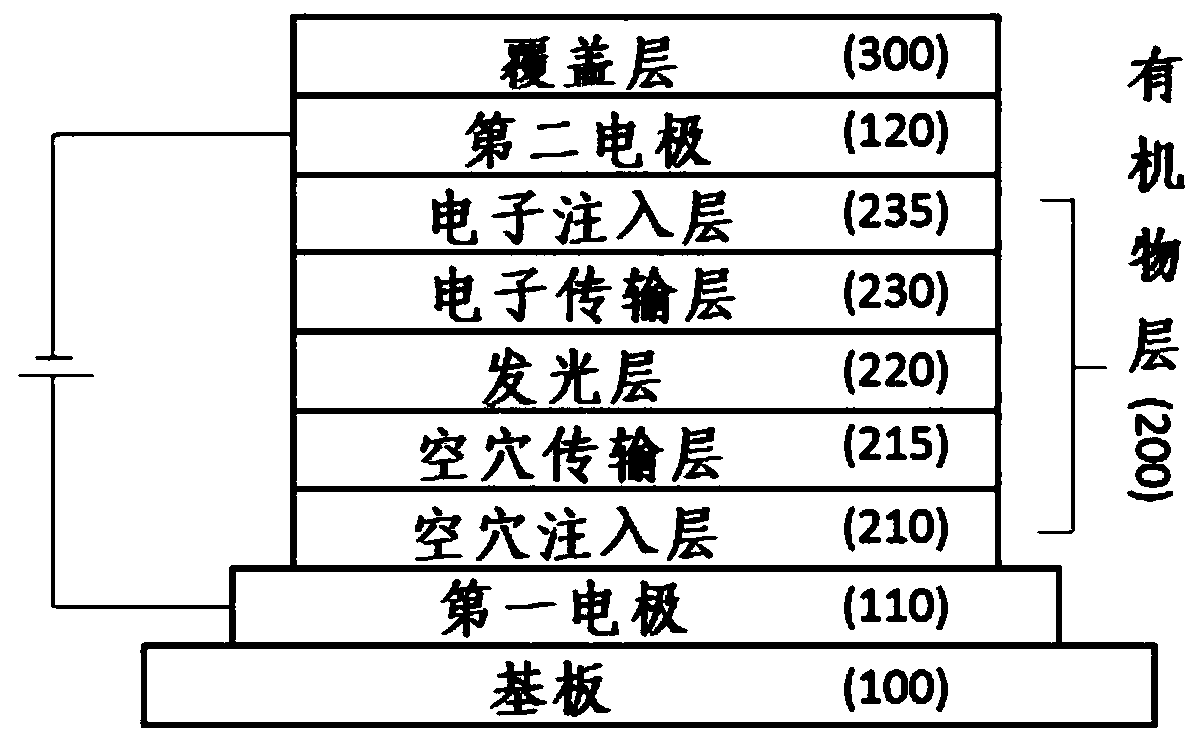
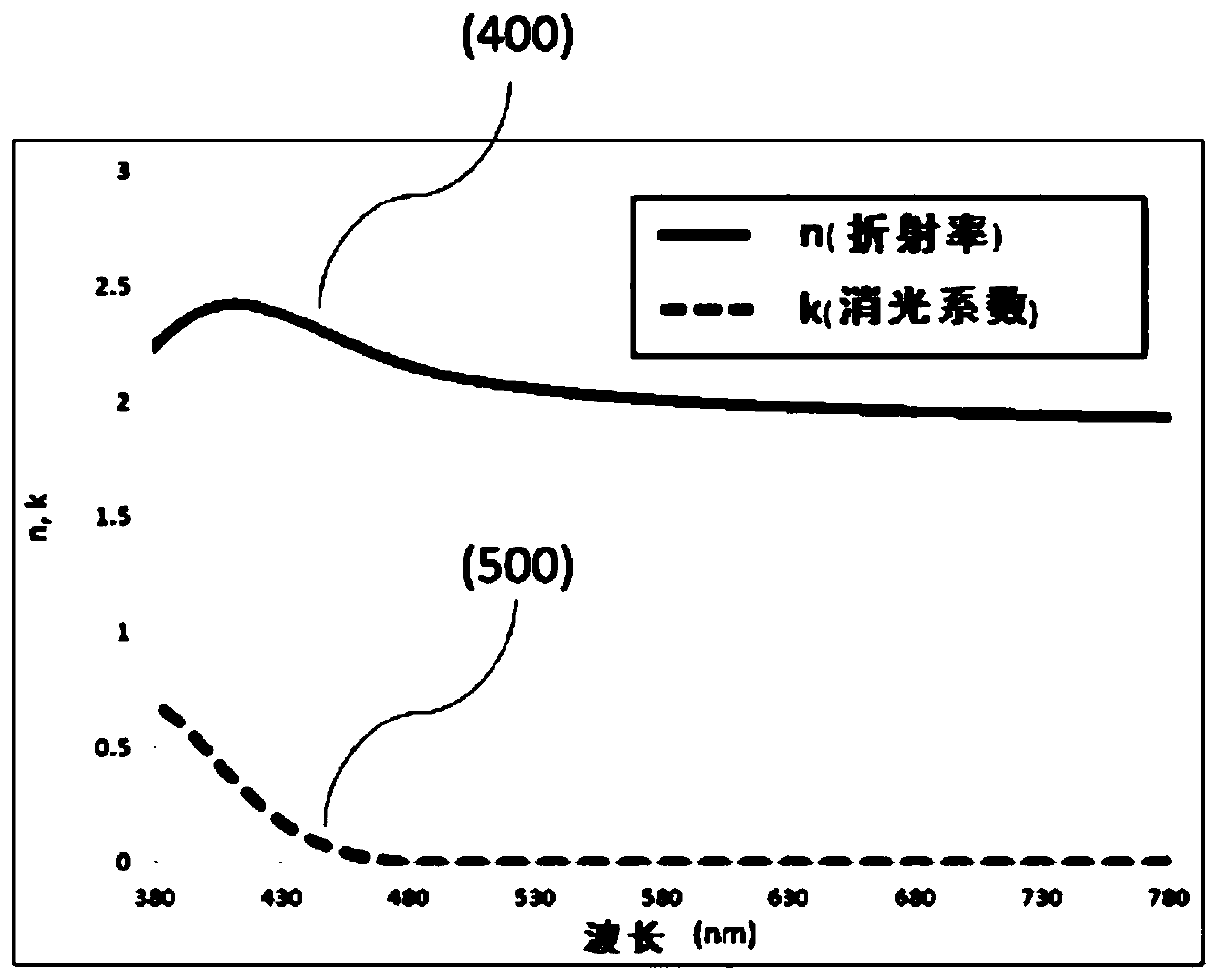
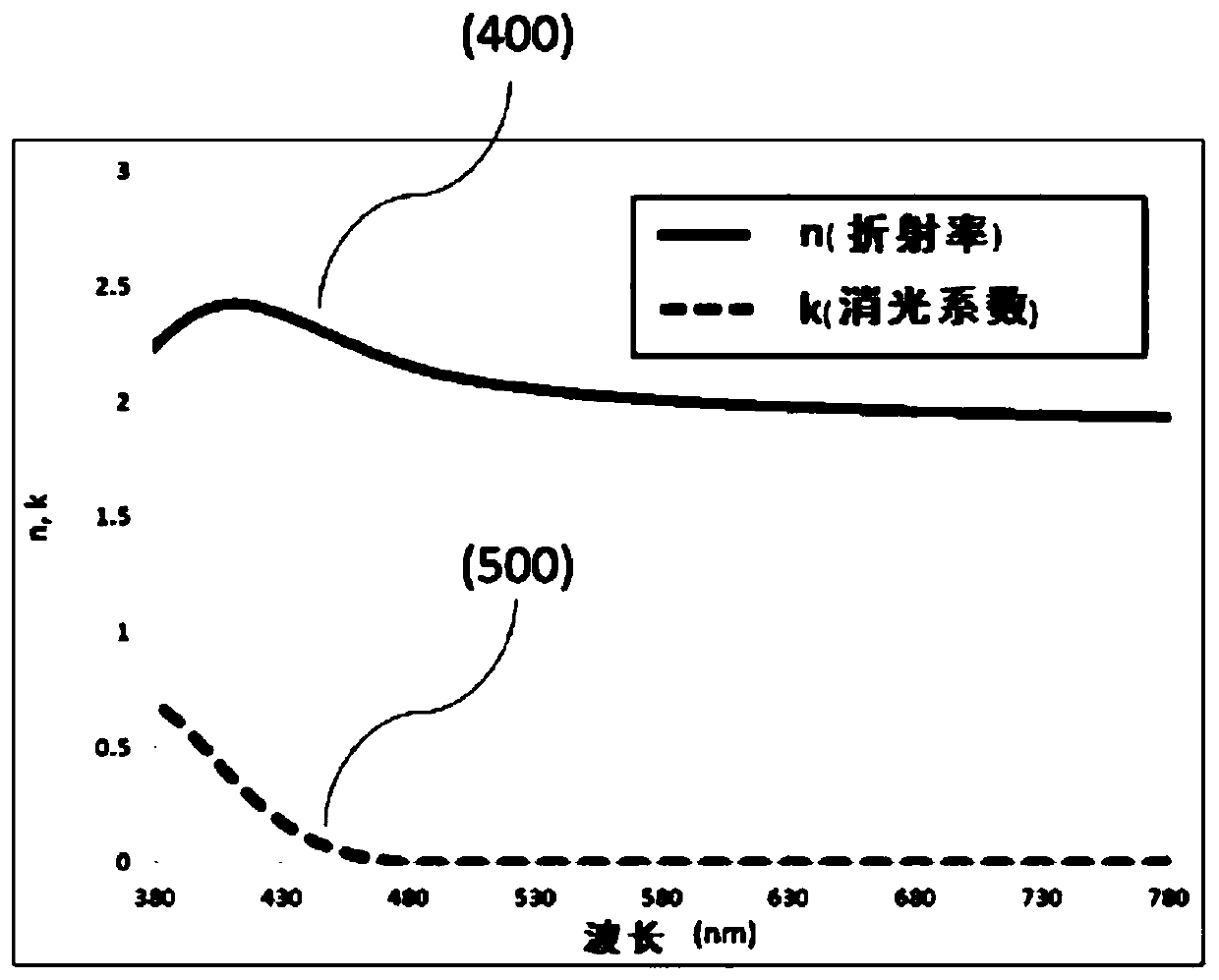
Aryl amine derivatieves and organic electroluminescent device including the same
A technology of derivatives and arylamines, which is applied in the field of organic electroluminescent display devices, can solve problems such as deviation, and achieve the effects of reducing half-value width, improving viewing angle characteristics, and maintaining efficiency and lifespan
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Synthetic example 1
[0131] Intermediate Synthesis Example 1: Synthesis of Intermediate (2)
[0132]
[0133] Add 10.0 g (0.092 mol) of 2-aminophenol, 16.9 g (0.092 mol) of 4-bromobenzaldehyde and 114 mL of ethanol, and stir at room temperature for 6 hours. After the reaction, the solvent was distilled under reduced pressure and dried to obtain a crude intermediate (1). The purification process was omitted and the following reactions were carried out.
[0134] After the intermediate (1) was dissolved in 370 mL of dichloromethane, 2,3-dichloro-5,6-dicyano-p-benzoquinone (2,3-Dichloro-5,6 -dicyano-p-benzoquinone, DDQ) 22.8 g (0.101 mol). After stirring for a whole day, purification was performed by column chromatography (DCM). Solidification was carried out with methanol to obtain 30.5 g (yield: 94.4%) of a white solid compound (intermediate body (2)).
[0135] Intermediate Synthesis Example 2: Synthesis of Intermediate (4)
[0136]
[0137] Add 10.0 g (76.88 mmol) of 2-aminobenzeneth...
Synthetic example 10
[0168] Intermediate Synthesis Example 10: Synthesis of Intermediate (19)
[0169]
[0170] Add intermediate (15) 20.0g (51.24mmol), ethylamine (Ethylamine) 6.9g (153.73mmol), Pd (dba) in 500mL single-necked flask 2 1.4g(2.56mmol), 50%P(t-Bu) 3 After mixing 2.0 g (5.12 mmol), 12.3 g (128.11 mmol) of NaOtBu (128.11 mmol), and 200 mL of toluene (Toluene), it was refluxed. After the reaction, it was cooled to normal temperature, solidified with MeOH, and filtered. The solid was purified by silica gel column chromatography (MC:HEX), solidified with EA, and filtered to obtain 8.2 g (yield: 45.1%) of a compound (intermediate (19)) as a yellow solid.
Synthetic example 11
[0171] Intermediate Synthesis Example 11: Synthesis of Intermediate (20)
[0172]
[0173] In a 500mL single-necked flask, add intermediate (2) 20.0g (72.96mmol), ethylamine (Ethylamine) 6.5g (145.92mmol), Pd (dba) 2 2.1g (3.65mmol), 50%P(t-Bu) 3 After mixing 1.5 g (3.65 mmol), 14.0 g (145.92 mmol) of NaOtBu (145.92 mmol), and 200 mL of toluene (Toluene), it was refluxed. After the reaction, it was cooled to normal temperature, solidified with MeOH, and filtered. The solid was purified by silica gel column chromatography (MC:HEX), solidified with EA, and filtered to obtain 7.2 g (yield: 41.0%) of a compound (intermediate (20)) as a yellow solid.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com