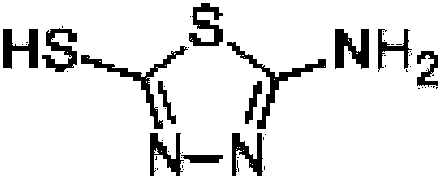
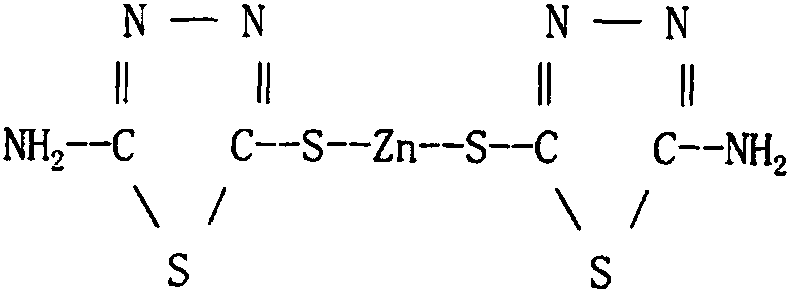Zinc thiazole preparation method used for reducing impurity content of zinc thiazole
A technology of thiazole zinc and thiadiazole, which is applied in the field of preparation of thiazole zinc to reduce the impurity content in thiazole zinc, can solve problems such as difficulty in industrial implementation, difficulty in meeting production requirements, complexity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1: prepare thiazole zinc by high pH value thiadiazole sodium solution
[0038] The thiadiazole salt solution is prepared by using different initial concentrations of sodium hydroxide solution. The specific method is to dissolve sodium hydroxide in water and add water to 100ml, then add a certain amount of solid thiadiazole, and heat to 80°C for 30 minutes to obtain thiadiazole salt solution. Diazol sodium solution.
[0039] The pH value of the final solution of the thiadiazole sodium solution is controlled by adjusting the addition amount of thiadiazole, and the pH value is adjusted at 12-15.
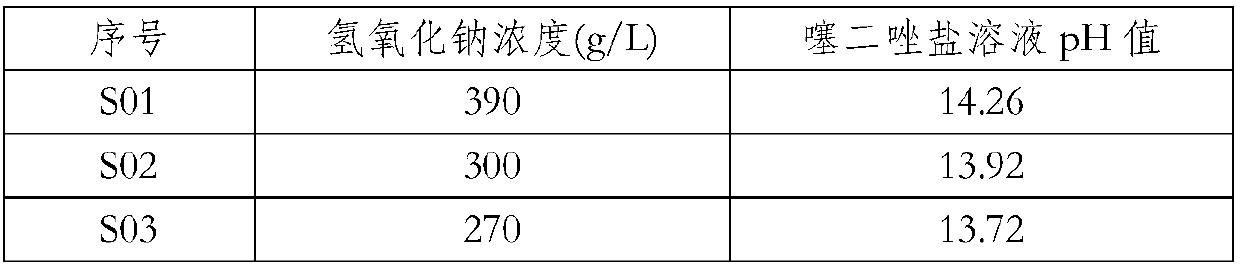
[0040] Specifically, the different sodium hydroxide concentrations and the pH value of the final thiadiazole sodium solution are as shown in Table 1.
[0041] The preparation of table 1 high concentration thiadiazole sodium solution
[0042]
[0043]
[0044] Apply above-mentioned thiadiazole sodium solution to prepare thiazole zinc, zinc salt adopts zinc sulfa...
Embodiment 2
[0051] Embodiment 2: low pH thiadiazole sodium solution prepares thiazole zinc
[0052] Prepare thiazole zinc in the same way as in Example 1. When adjusting the pH value of the thiadiazole sodium solution, the pH value of the final solution of the thiadiazole salt solution is controlled between 8-13 by adjusting the amount of thiadiazole added.
[0053] Specifically, the pH values of different sodium hydroxide concentrations and the final thiadiazole sodium solution are shown in Table 3 below.
[0054] The preparation of table 3 high concentration thiadiazole sodium solution
[0055] serial number Sodium hydroxide concentration (g / L) Thiadiazole Sodium Solution pH L01 60 8.02 L02 70 8.14 L03 80 8.54 L04 90 9.32 L05 100 10.35 L06 110 11.43 L07 120 12.54
[0056] Apply above-mentioned thiadiazole sodium solution to prepare thiazole zinc, zinc salt adopts zinc sulfate, the molar concentration of thiadiazole sodium sol...
Embodiment 3
[0061] Embodiment 3: other condition control in preparing thiazole zinc
[0062] In the preparation process of embodiment 1 and embodiment 2, thiadiazole sodium solution is replaced by other thiadiazole alkaline solutions, zinc sulfate solution is replaced by other zinc salt solutions, by controlling the pH of thiadiazole salt solution value and the pH value in the reaction system to detect the content of impurity zinc hydroxide and impurity thiadiazole in the zinc thiazole product, the relationship between its pH value and impurity content and the relationship with the yield are the same as in Example 1 and 2 results trend.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com