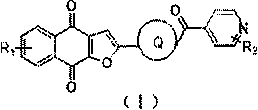
Novel small molecule compound, preparation method and application thereof in preparation of mycobacterium drugs for resisting drug-resistant mycobacterium tuberculosis and like
Technology of a small molecule compound, Mycobacterium tuberculosis, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
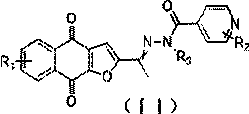
[0043] Embodiment 1: ( Z / E )- N' Preparation of -(1-(4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-2-yl)ethylidene)isonicotinic acid hydrazide (1-a)
[0044] .
[0045] Add 200mg (0.833mmol) 2-acetylfuro[2,3-b]-4,9-naphthoquinone, 171mg (1.250mmol) isoniazid, AcOH / EtOH=0.5:100 to a 10ml sealed reaction tube Solvent 3ml, stirred at 78°C for 15h. During the reaction, a yellow solid precipitated out. After the reaction, it was cooled to room temperature, filtered with suction, and washed with absolute ethanol several times to obtain 205 mg of a yellow solid with a yield of 68.5%. 1 H NMR (400 MHz, DMSO) δ 11.23 (s, 1H), 8.79 (s, 2H), 8.13 (s, 2H), 7.91 (s, 2H), 7.82 (s, 2H), 7.64 (s, 1H) , 2.45 (s, 3H). MS (m / z) 360.0911.
Embodiment 2
[0046] Example 2: N' Preparation of -(1-(4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-2-yl)ethyl)isonicotinic acid hydrazide (1-b)
[0047] .
[0048]In a sealed 25ml reaction tube, add 800mg (3.333mmol) 2-acetylfuro[2,3-b]-4,9-naphthoquinone to 8ml methanol solution, add 380mg (9.999mmol) sodium borohydride, and seal Stir at room temperature for 3 hours, stir in air for 10 minutes, add distilled water drop by drop while stirring, precipitate a yellow solid, filter with suction, and dry to obtain 2-(1-hydroxyethyl)naphtho[2,3-b]furan- 771 mg of 4,9-diketone yellow solid, yield: 95.01%. In a 25ml pear-shaped flask, add 750mg (3.074mmol) of 2-(1-bromoethyl)naphtho[2,3-b]furan-4,9-dione into 8ml of dichloromethane solution, and stir for 5min. Add PBr dropwise 3 873.6ul (9.222mmol), stirred at room temperature for 2h, washed the reaction solution with distilled water several times, and spin-dried dichloromethane to obtain 2-(1-bromoethyl)naphtho[2,3-b]furan-4,9 - Diketone yello...
Embodiment 3
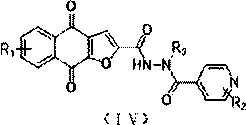
[0049] Example 3: N' Preparation of -(4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-2-carbonyl)isonicotinic acid hydrazide (1-c)
[0050] .
[0051] In a sealed 10ml reaction tube, add 90mg (0.368mmol) of 4,9-dioxo-4,9-dihydronaphtho[2,3-b]furan-2-carboxylic acid and 209mg (0.550mmol) of HATU into 1.5ml of DMF solution, stirred at room temperature for 5min, added 56mg (0.55mmol) of triethylamine, and then added 75mg (0.55mmol) of isoniazid, and reacted at room temperature for 2h, while stirring, added H 2 O, a yellow solid was precipitated, filtered by suction, and spin-dried to obtain 40 mg of a yellow solid, the yield: 30.10%. 1 H NMR (400 MHz, DMSO) δ11.25 (s, 1H), 11.19 (s, 1H), 8.95 – 8.88 (m, 2H), 8.21 – 8.12 (m, 2H), 8.04 – 7.99 (m, 2H) , 7.97 – 7.90 (m, 2H), 7.84 (s, 1H). MS (m / z) 362.0701.
[0052] In order to evaluate the antibacterial activity of the compounds of the present invention, the compounds of the present invention were tested for their pharmacological act...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com