Application of inactivated plasmodium to preparing medicine for treating cancer
A technology of Plasmodium and Plasmodium falciparum, applied in the field of biomedicine, can solve the problems of limited research or new applications of Plasmodium, and achieve the effects of enhancing anti-tumor immune response, high safety, and prolonging the survival period
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of Inactivated Plasmodium Suspension and Red Blood Cell Suspension
[0037] (1) Preparation of inactivated Plasmodium suspension
[0038] The Plasmodium strain used was Plasmodium falciparum PfNF54 strain, which was derived from The Malaria Research and Reference Reagent Resource Center (MR4), and the Plasmodium was continuously cultured according to the existing mature conventional method.
[0039] The specific method is:
[0040] (I) Transfer the Plasmodium cultured in vitro (infection rate>8%, in the stage of large trophozoite and schizont) to a centrifuge tube, centrifuge at 300g for 5min, and discard the supernatant;
[0041] (II) Add twice the volume of 1640 medium to resuspend, centrifuge at 300g for 5 minutes, discard the supernatant, take out a small part of the blood smear, and calculate the infection rate to be above 90%.
[0042] (Ⅲ) Add 3 mL of 70% Percoll separation solution to a 15 mL centrifuge tube, and slowly and carefully add 1 mL of the a...
Embodiment 2
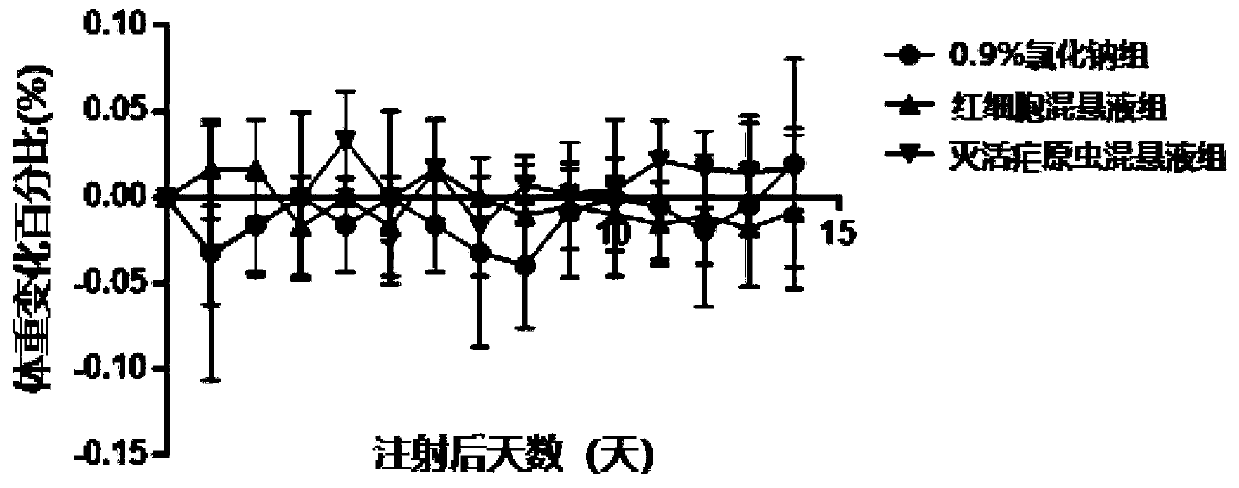
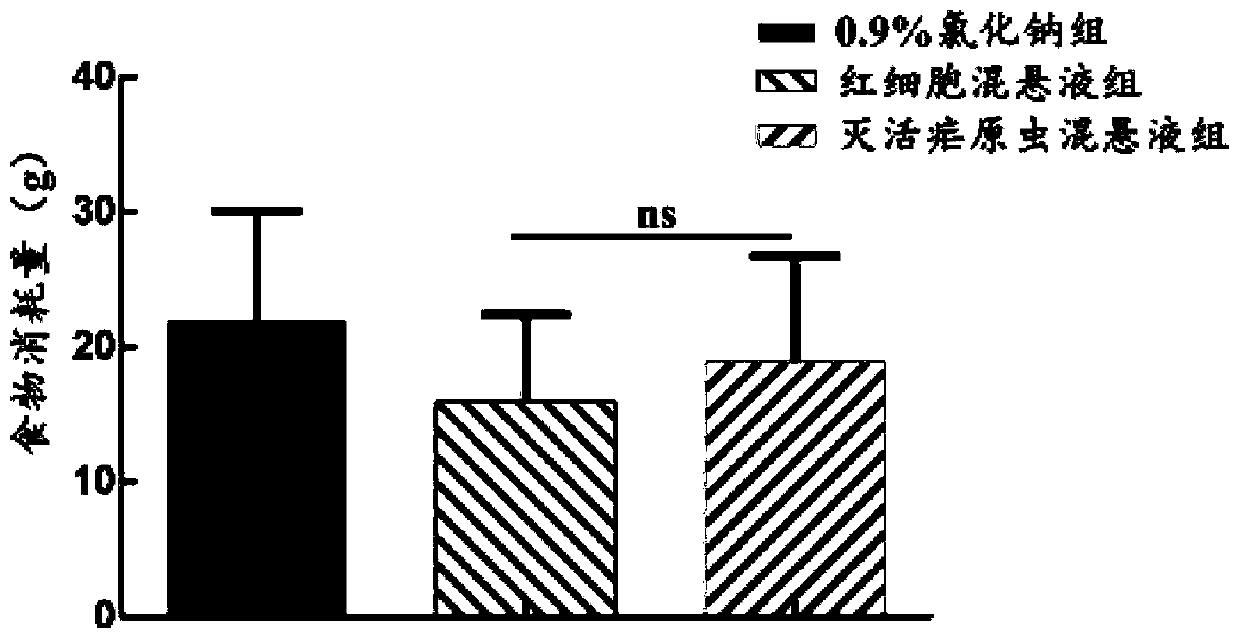
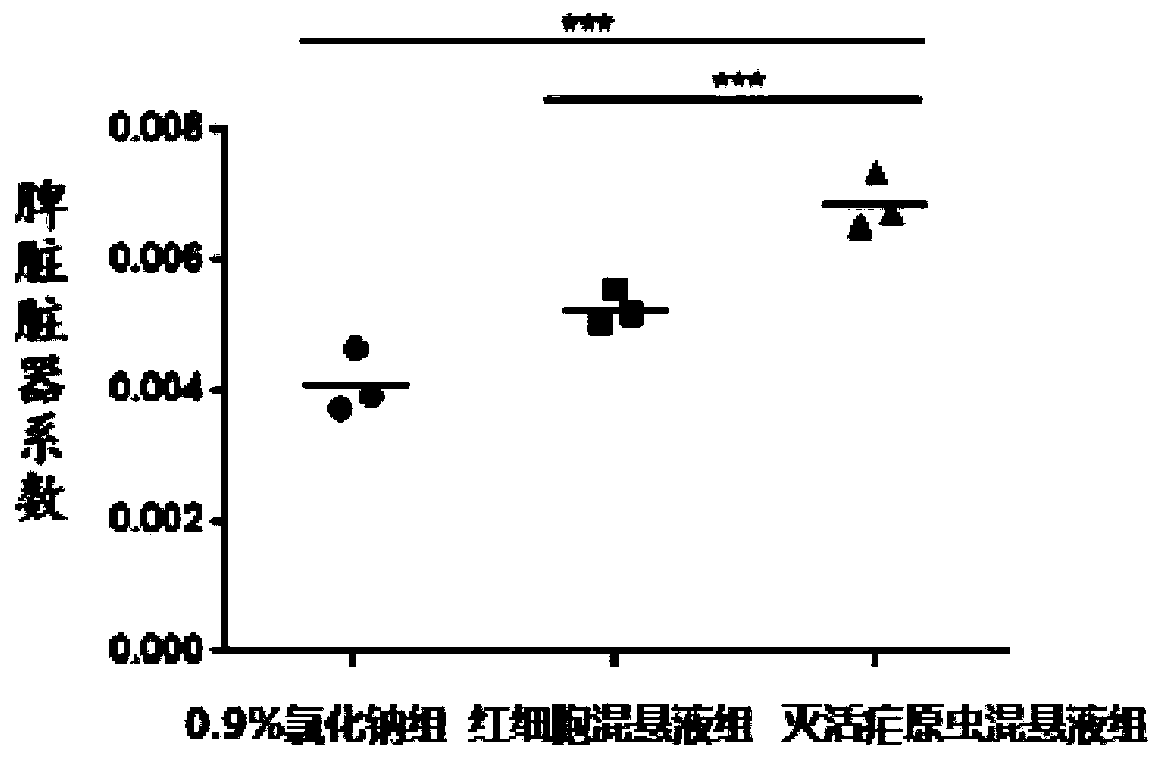
[0049] Safety Evaluation Test
[0050] (1) Experimental animals and materials: C57BL / 6J mice, female, 4 weeks old, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.; inactivated Plasmodium Pf NF54 suspension; broken red blood cell suspension, Human type O red blood cells; 75% ethanol; normal saline; 1ml insulin syringe, etc.
[0051] (2) Raising conditions of experimental animals: All mice were free to forage for food and drink water, room temperature (23±2)°C, and were raised in Guangzhou Yuansheng Pharmaceutical Technology Co., Ltd.; the feed and water were autoclaved, and all experimental feeding The process and experiments were carried out in accordance with relevant experimental standards.
[0052] (3) Experimental steps:
[0053] (I) Take 9 C57BL / 6J mice and divide them into 0.9% sodium chloride group, erythrocyte suspension group and inactivated malaria parasite suspension group, totally 3 groups. Inject normal saline, red blood cell suspe...
Embodiment 3
[0067] Pharmacodynamic evaluation test on mouse model of colon cancer
[0068] (1) Experimental animals and materials: Balb / c mice, female, 4 weeks old, purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.; inactivated Plasmodium suspension; red blood cell suspension; mouse colon cancer Cell C26 was obtained from the American Type Culture Collection.
[0069] (2) Raising conditions of experimental animals: All mice were free to forage for food and drink water, room temperature (23±2)°C, and were raised in Guangzhou Yuansheng Pharmaceutical Technology Co., Ltd.; the feed and water were autoclaved, and all experimental feeding The process and experiments were carried out in accordance with relevant experimental standards.
[0070] (3) Experimental steps:
[0071] (I) get 30 Balb / c mice, divide into 0.9% sodium chloride group; Red blood cell suspension group; Inactivated Plasmodium suspension group, altogether 3 groups;
[0072] (II) C26 cells were cul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com