Porphyrin-based metal organic framework material for CO2 cyclization catalytic reaction and preparation method thereof
A catalytic reaction and organic framework technology, applied in the field of nanomaterials, can solve the problems of loss of activity and agglomeration, and achieve the effects of high reusability, strong stability and high catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Synthesis of flower-like PCN-222
[0025] Add 20mg ZrCl in 4mL DMF 4 ·6H 2 O, 100 μL triethylamine, 480 μL glacial acetic acid, and 100 μL HO 2 O, after ultrasonic dispersion for 5 min, add 20 mg of Ni-TCPP to the above solution, and stir magnetically for 10 min at room temperature. The resulting homogeneous solution was transferred to a 24 mL Teflon-lined stainless steel autoclave and then heated at 130 °C for 12 h. After cooling to room temperature, it was separated by centrifugation at 8000 rpm for 8 minutes and further purified several times with ethanol.
Embodiment 2
[0033] PCN-222 activation process
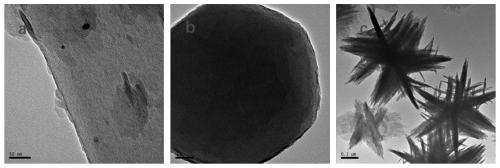
[0034] The synthesized sample (~100 mg) and 1.5 mL of 8M HCl were added to 40 mL of DMF, and the DMF suspension was stirred at 120 °C for 12 h to remove unreacted starting ligands, inorganic substances and conditioning reagents. Subsequently, the extract was decanted carefully and the product was dispersed in fresh acetone for 24 hours to exchange and remove DMF. After removing acetone by centrifugation, the samples were activated by vacuum drying at 120 °C for 24 hours. Then the active samples were used for XRD, BET, SEM, TEM characterization and catalytic reaction test.
Embodiment 3
[0036] Cyclization assay
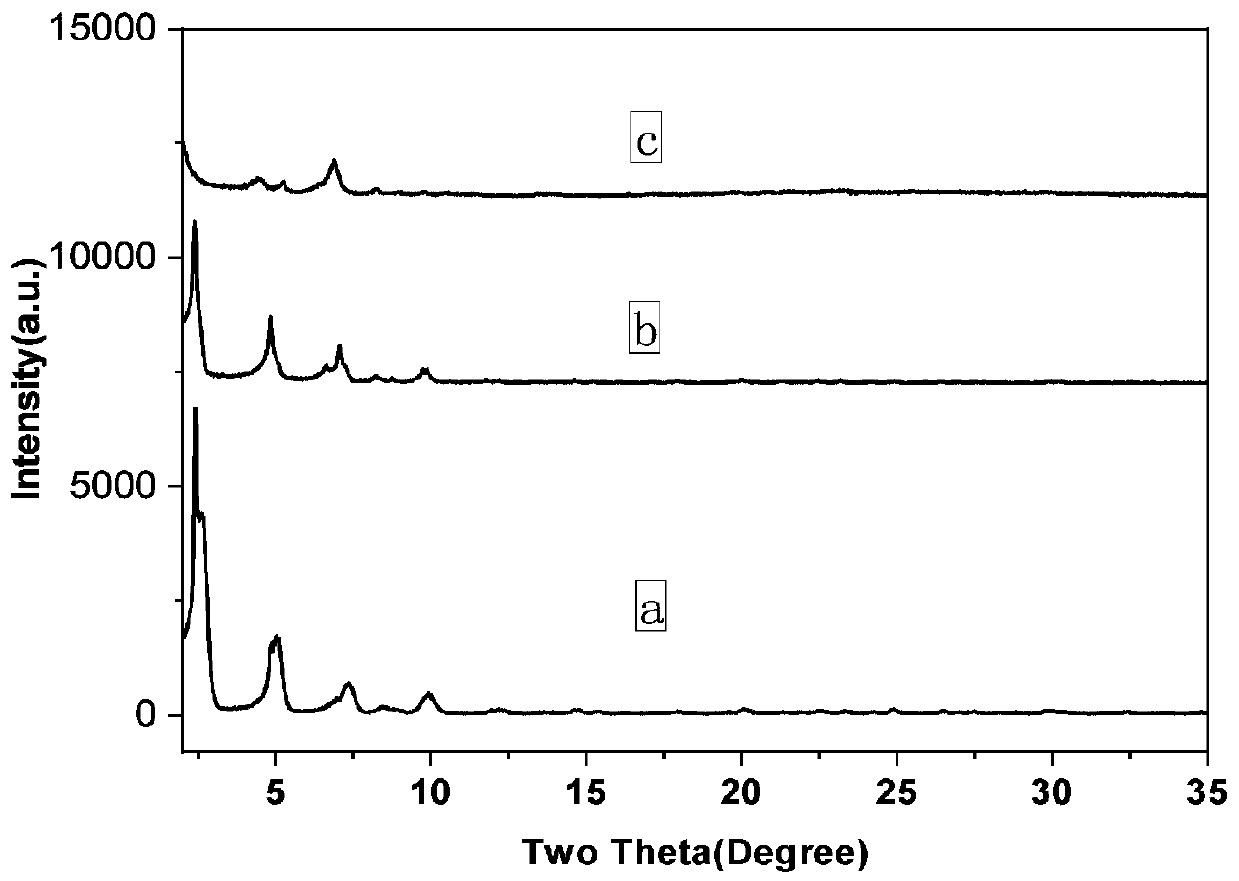
[0037] The PCN-222 with different morphology prepared in Example 1 and Comparative Example 1-2 was selected for CO 2 The cyclization reaction was measured and compared. The flower-shaped PCN-222 was subjected to repeated utilization experiments.
[0038] Dissolve 20mg of rod-shaped PCN-222 and 281mg of TABA in 2ml of styrene oxide, and pass through CO 2, placed in an 80°C oil bath for 20h. Take 50 μL of the reacted solution, dilute it in 1 mL of ethyl acetate, centrifuge, take the supernatant for gas chromatography analysis, and calculate the yield.
[0039] Dissolve 20mg of hexagonal PCN-222 and 281mg of TABA in 2ml of styrene oxide, and pass through CO 2 , placed in an oil bath at 80°C for 20 hours. Take 50 μL of the reacted solution, dilute it in 1 mL of ethyl acetate, centrifuge, take the supernatant for gas chromatography analysis, and calculate the yield.
[0040] Dissolve 20 mg of flower-like PCN-222 and 281 mg of TABA in 2 mL of styrene...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com