Method for photodegradation of organic pollutants in water by black carbon
A technology of organic pollutants and photodegradation, applied in the field of water pollution degradation, can solve the problems of unstable chemical properties, non-pollutants, low catalytic efficiency, etc., and achieve the effects of mild reaction conditions, simple reaction operation, and important environmental significance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Fill the chromatographically pure n-hexane into the fuel pool, ignite the n-hexane through the wick extending into the fuel pool to generate flame, and enter the combustion chamber through precise control N 2 And O 2 The content controls the combustion-oxygen ratio of the n-hexane combustion process. In this experiment, the combustion-oxygen ratio is controlled at 0.18. Collect the black carbon with a 10 cm * 10 cm quartz plate 4 cm above the flame, scrape the collected black carbon, and place it in a sealed reagent bottle for later use.
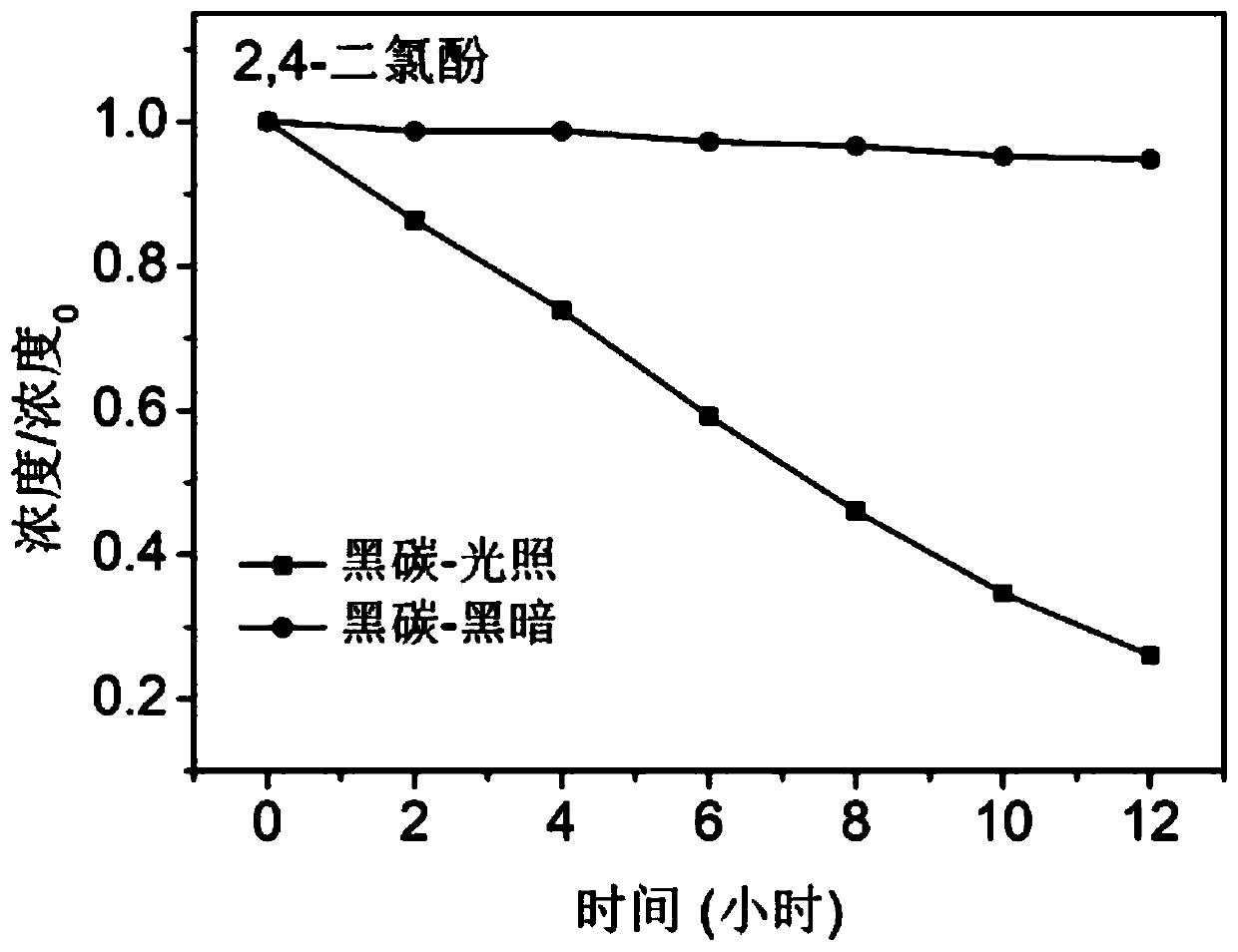
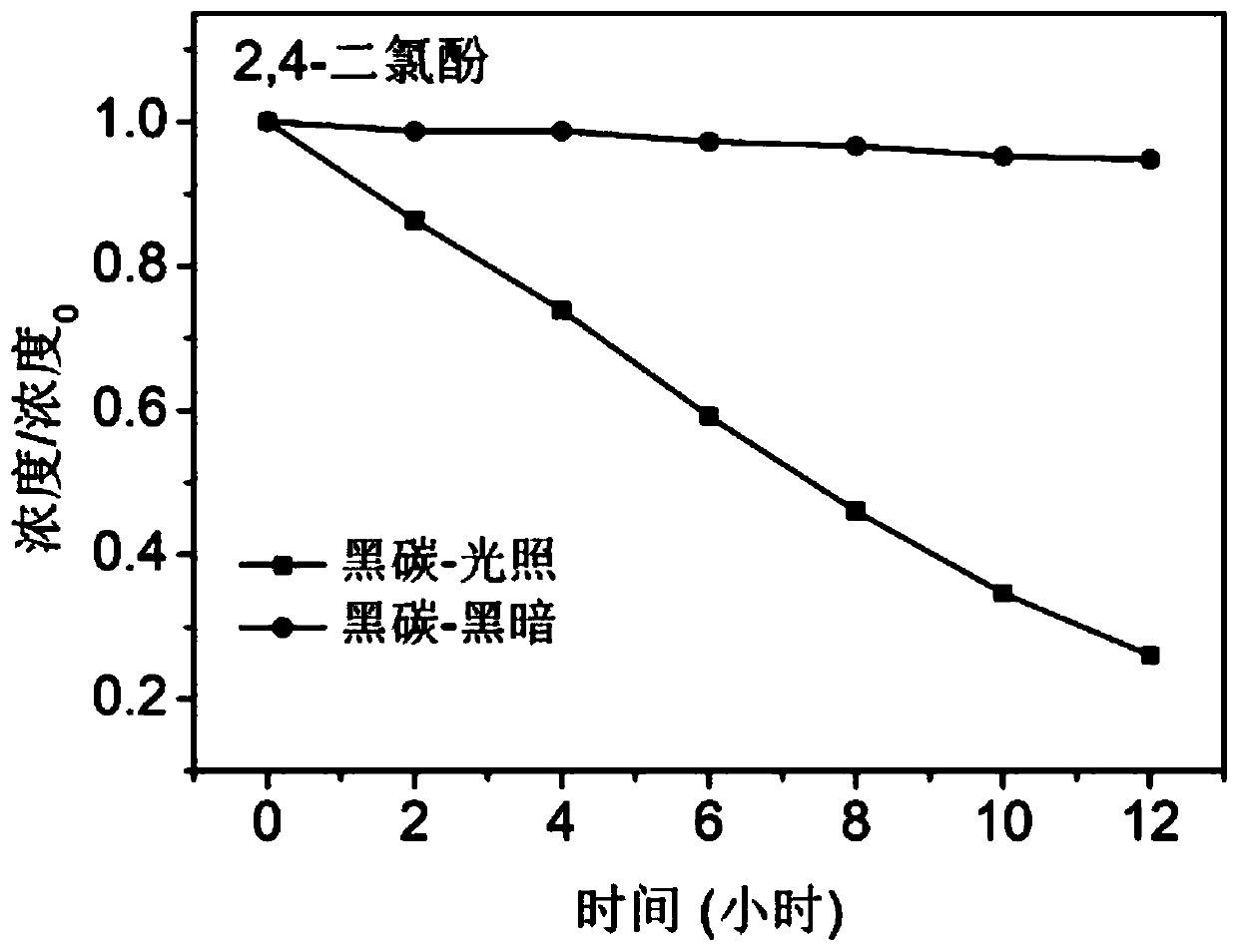
[0032] The black carbon prepared above is added to a temperature-controlled transparent photochemical reactor containing 2,4-dichlorophenol aqueous solution. Among them, the black carbon concentration is 0.33mg / ml, and the initial concentration of 2,4-dichlorophenol is 5*10 -5 M, then sonicate the photoreactor for 5 minutes so that the suspension is evenly mixed. After the end of the ultrasound, the reactor was stirred (20 revolutions / mi...
Embodiment 2
[0034] Fill the chromatographically pure n-hexane into the fuel pool, ignite the n-hexane through the wick extending into the fuel pool to generate flame, and enter the combustion chamber through precise control N 2 And O 2 The content controls the combustion-oxygen ratio of the n-hexane combustion process. In this experiment, the combustion-oxygen ratio is controlled at 0.18. Collect the black carbon with a 10 cm * 10 cm quartz plate 4 cm above the flame, scrape the collected black carbon, and place it in a sealed reagent bottle for later use.
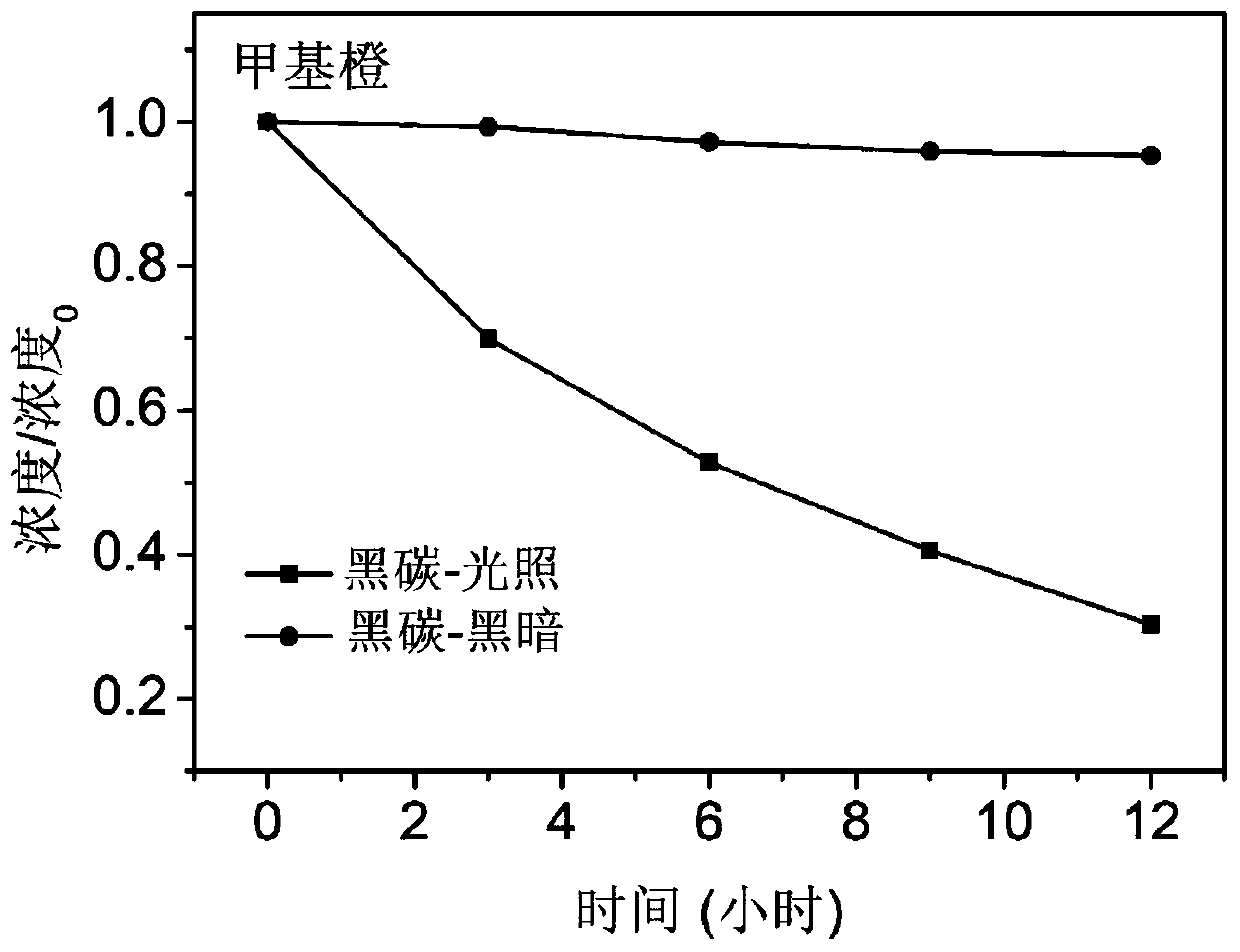
[0035] The black carbon prepared above is added to a temperature-controlled transparent photochemical reactor containing a methyl orange aqueous solution. Among them, the black carbon concentration is 0.33mg / ml, and the initial concentration of methyl orange is 4*10 -5 M, then sonicate the photoreactor for 5 minutes so that the suspension is evenly mixed. After the end of the ultrasound, the reactor was stirred (20 revolutions / min) under...
Embodiment 3
[0037] Fill the chromatographically pure n-hexane into the fuel pool, ignite the n-hexane through the wick extending into the fuel pool to generate flame, and enter the combustion chamber through precise control N 2 And O 2 The content controls the combustion-oxygen ratio of the n-hexane combustion process. In this experiment, the combustion-oxygen ratio is controlled at 0.18. Collect the black carbon with a 10 cm * 10 cm quartz plate 4 cm above the flame, scrape the collected black carbon, and place it in a sealed reagent bottle for later use.
[0038] The black carbon prepared above is added to a temperature-controlled transparent photochemical reactor containing a bisphenol A aqueous solution. Among them, the black carbon concentration is 0.33mg / ml, and the initial concentration of bisphenol A is 2*10 -5 M, then sonicate the photoreactor for 5 minutes so that the suspension is uniformly mixed. After the end of the ultrasound, the reactor was stirred (20 revolutions / min) under ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com