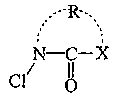Algicidal/microbicidal agent and algicidal/microbicidal method
A technology of algicides and fungicides, applied in the direction of fungicides, herbicides, algicides, biocides, etc., can solve the problems of evaluation, recording, unsatisfactory, etc., and achieve low risk and low corrosion Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
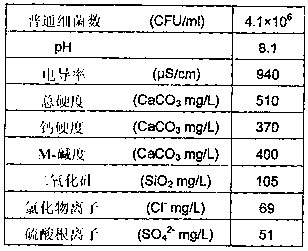
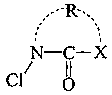
[0064] In Example 1, 6.8 g of 2-pyrrolidone [reagent, manufactured by Kanto Chemical Co., Ltd.] was added to 100 g of chloroform [reagent, manufactured by Kanto Chemical Co., Ltd.], and trichloroisocyanuric acid [ Reagent, manufactured by Tokyo Chemical Industry Co., Ltd.] 9.3 g. After stirring the resulting white slurry for 6 hours, suction filtration was performed. The filtrate was concentrated with a rotary evaporator and cooled with ice to obtain 9.4 g of white crystals of N-chloro-2-pyrrolidone (98.4% yield, physical properties shown below).
[0065] 1 H-NMR (CDCl 3 ,δ):
[0066] 2.28(m, 4H), 4.21(t, 2H)
[0067] IR (KBr, cm -1 ):
[0068] 2968, 1708, 1385, 1254, 1135, 820.
[0069] 4.6 g of acetic acid and 86.0 g of water were added to 9.4 g of white crystals of this N-chloro-2-pyrrolidone to prepare 100 g of an aqueous N-chloro-2-pyrrolidone solution having an available chlorine concentration of 5.5%. In addition, according to the DPD-ammonium iron (II) sulfate ...
Embodiment 2
[0070] In Example 2, 7.6 g of 2-piperidone [reagent, manufactured by Kanto Chemical Co., Ltd.] and 42.8 g of water were mixed, 4.6 g of acetic acid [reagent, manufactured by Kanto Chemical Co., Ltd.] was added, and the concentration of available chlorine was added to the resulting aqueous solution. 100 g of an N-chloro-2-piperidone aqueous solution having an available chlorine concentration of 5.4% was prepared from 45.0 g of a 12% sodium hypochlorite solution [12% available chlorine concentration, manufactured by Yayoi Sangyo Co., Ltd.]. According to the DPD-ammonium iron (II) sulfate titration method, the chlorine form of the aqueous solution is a combined type.
Embodiment 3
[0071] In Example 3, 7.9 g of ε-caprolactam [a reagent, manufactured by Kanto Chemical Co., Ltd.] was added to 100 g of chloroform, and 8.1 g of trichloroisocyanuric acid was added over 90 minutes under ice cooling. After the resulting white slurry was stirred for 6 hours, suction filtration was performed, and the filtrate was concentrated with a rotary evaporator to obtain 10.1 g of a light yellow transparent oil of N-chloro-ε-caprolactam (97.9% yield, physical properties shown below).
[0072] 1 H-NMR (CDCl 3 ,δ):
[0073] 1.78(br.s, 6H), 2.45(d, 2H), 3.21(t, 2H)
[0074] IR (NaCl, cm-1 ):
[0075] 2933, 2858, 1672, 1452, 1191, 980.
[0076] 10.0 g of γ-butyrolactone [reagent, manufactured by Kanto Chemical Co., Ltd.], 4.1 g of acetic acid, and 75.8 g of water were added to 10.1 g of the light yellow transparent oil of N-chloro-ε-caprolactam to prepare an available chlorine concentration of 4.7 % N-chloro-ε-caprolactam aqueous solution 100g. According to the DPD-ammoni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com