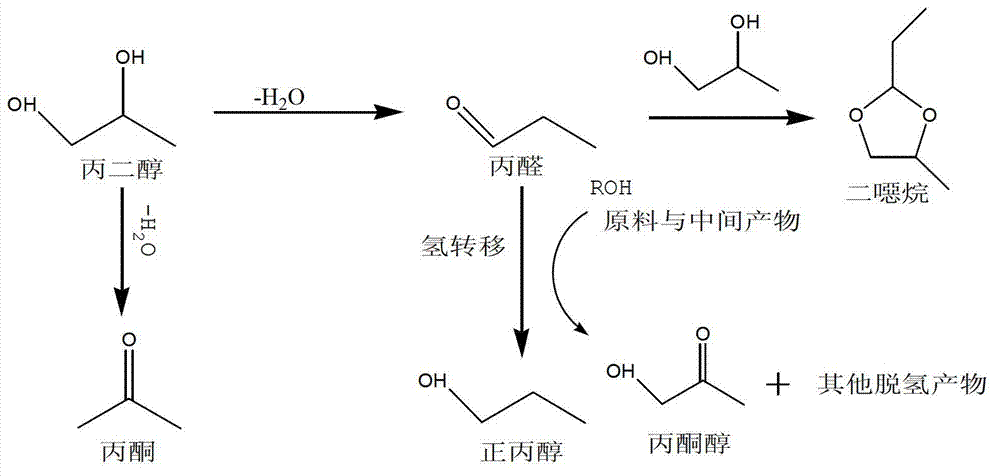
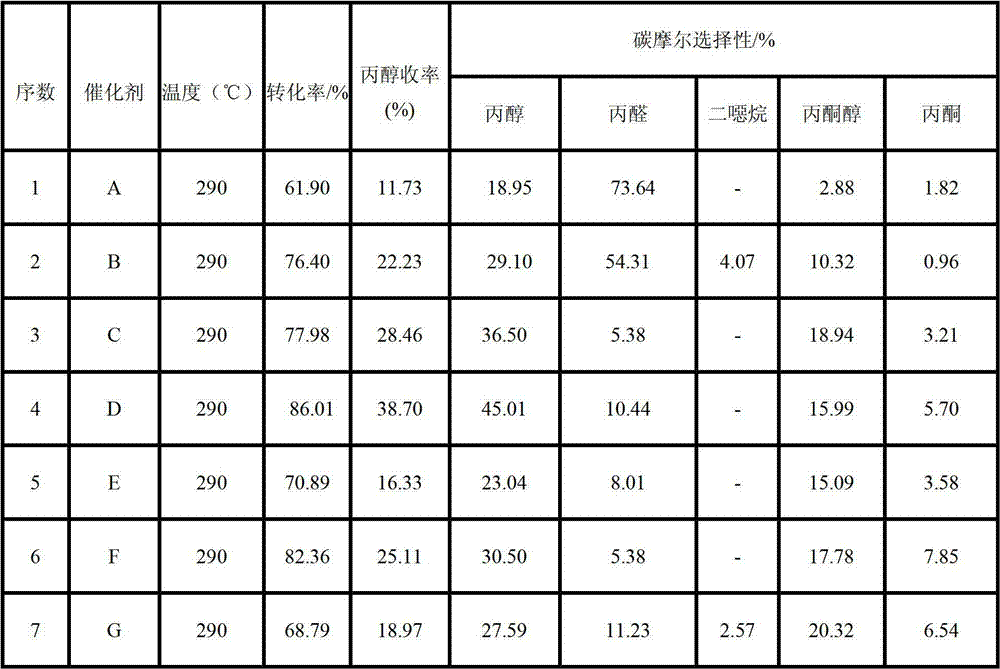
Method for preparing n-propanol from bio-base diol
A bio-based diol, n-propanol technology, applied in the chemical industry, can solve the problems of difficult product separation, long reaction time, complex catalyst preparation process, etc., and achieve the effect of easy reaction operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The preparation process of catalyst A is as follows: with the dried γ-Al 2 o 3 As a carrier, add a certain amount of ammonium metatungstate solution (the mass of W contained in ammonium metatungstate is about 30% of the carrier) by equal volume impregnation method, impregnate for 24 hours, transfer to an oven at 110°C for 24 hours, and dry at 600°C Catalyst samples were prepared after calcination for 4 h.
Embodiment 2
[0019] The preparation process of catalyst B is as follows: with the ZrO after drying 2 As a carrier, add a certain amount of ammonium metatungstate solution (the mass of W contained in ammonium metatungstate is about 30% of the carrier) by equal volume impregnation method, impregnate for 24 hours, transfer to an oven at 110°C for 24 hours, and dry at 600°C Catalyst samples were prepared after calcination for 4 h.
Embodiment 3
[0021] The preparation process of catalyst C is as follows: Adopt co-precipitation method to prepare Zr(OH) 4 ·xH 2 O, with dried Zr(OH) 4 ·xH 2 O was used as a carrier, and a certain amount of niobium oxalate solution (adjusted to pH ≤ 1 with nitric acid) was added by equal volume impregnation method, impregnated for 24 h, transferred to an oven at 110 °C for 24 h, and calcined at 600 °C for 4 h to prepare catalyst samples. (The mass of Nb contained in niobium oxalate solution is about 1.6% of the support).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com