Method for preparing methanoic acid by catalytic hydrogenation of nanometer porous nickel material
A nanoporous nickel and catalytic hydrogenation technology, applied in chemical instruments and methods, preparation of organic compounds, carboxylate preparation, etc., can solve the problems of harmful environment, high cost, complex production process of precious metal catalysts, etc., and reduce CO2 emissions , good selective effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
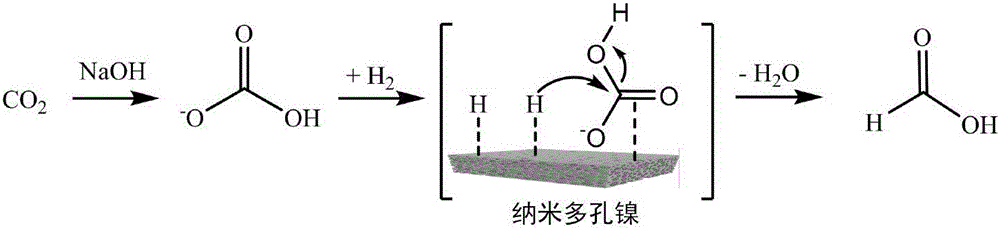
[0024] This embodiment relates to a nanoporous nickel material for catalytic hydrogenation of CO 2 The method for preparing formic acid, reaction equation is as follows:
[0025]
[0026] The method comprises the steps of:
[0027] CO 2 (1mmol), nanoporous nickel material (10mg) is packed into Teflon liner (volume is 100mL) autoclave, add the water that filling rate is 10% and make solvent, regulate above-mentioned solution pH with sodium hydroxide to be 8.3, then Reactor sealed. The 3MPa hydrogen required for the reaction is charged, the reaction temperature is 200°C, and the reaction time is 120 minutes. After the reaction is completed, the mixture is taken out and filtered to obtain formate. The obtained formate can be obtained by acid hydrolysis and distillation to obtain formic acid.
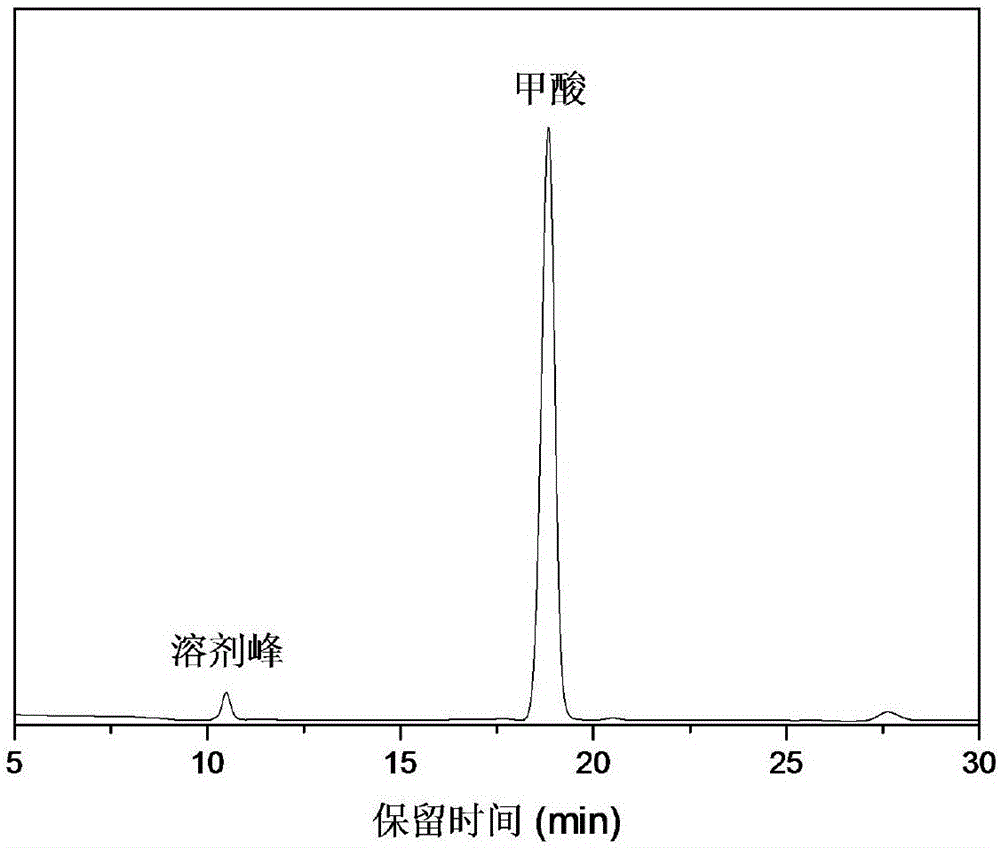
[0028] The reacted samples were sequentially analyzed qualitatively and quantitatively. Qualitative analysis showed that if figure 1 As shown, formic acid is the main product with ...
Embodiment 2
[0031] This embodiment relates to a nanoporous nickel material for catalytic hydrogenation of CO 2 The method for preparing formic acid, reaction equation is as follows:
[0032]
[0033] The method comprises the steps of:
[0034] CO 2 (1mmol), nanoporous nickel material (13mg) are packed into Teflon liner (volume is 100mL) autoclave, add the water that filling rate is 10% and make solvent, regulate above-mentioned solution pH with sodium hydroxide to be 8.3, then Reactor sealed. The 3MPa hydrogen required for the reaction was charged, the reaction temperature was 200°C, and the reaction time was 120 minutes. After the reaction was completed, the mixture was taken out and filtered to obtain a post-reaction sample. The obtained formate can be obtained by acid hydrolysis and distillation to obtain formic acid.
[0035] The reacted samples were sequentially analyzed qualitatively and quantitatively. Qualitative analysis showed that formic acid was the main product with a...
Embodiment 3
[0038] This embodiment relates to a kind of nanoporous nickel material catalytic hydrogenation NaHCO 3 The method for preparing formic acid, reaction equation is as follows:
[0039]
[0040] The method comprises the steps of:
[0041] NaHCO 3 (1mmol), nanoporous nickel material (13mg) is packed in Teflon liner (volume is 100mL) autoclave, adds the water of filling rate 10% and makes solvent, then reactor is sealed, is filled with the required 6MPa of reaction Hydrogen gas, the reaction temperature is 200° C., and the reaction time is 120 minutes. After the reaction is completed, the mixture is taken out and filtered to obtain a post-reaction sample. The obtained formate can be obtained by acid hydrolysis and distillation to obtain formic acid.
[0042] The reacted samples were sequentially analyzed qualitatively and quantitatively. Qualitative analysis showed that formic acid was the main product with a selectivity of >99%. Quantitative analysis showed that the highest...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com