Preparation method of 4-methylsulfonyl benzaldehyde
A technology of p-thymphenyl and benzaldehyde, which is applied in the field of preparation of p-thymphenyl benzaldehyde, can solve the problem of high cost, and achieve the effects of short steps, reduction of three waste emissions, and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
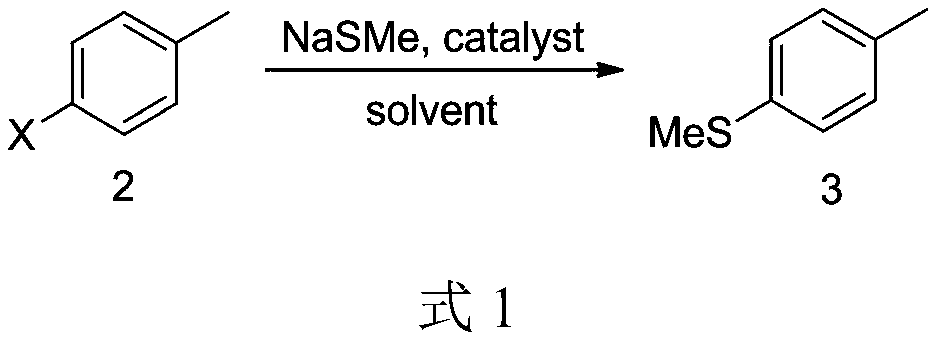
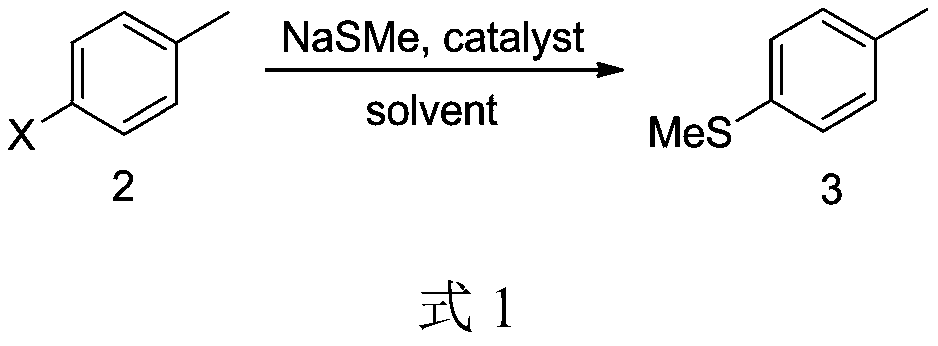
[0018] Embodiment 1: In the synthetic route shown in formula 1, X is a chlorine atom; the catalyst is CuCl; the solvent is dioxane.
[0019] Preparation of compound 3
[0020] Under nitrogen protection, compound 2 (12.6 g, 0.1 mol) was dissolved in 120 mL of dioxane in a 250 mL three-necked flask, and 30% sodium methyl mercaptide solution (35.0 g, 0.15 mol) and catalytic amount of CuCl (0.0003 mol, 0.03g). The reaction mixture was heated to reflux for 11 hours, cooled to room temperature, and the solvent was recovered under reduced pressure. 40 mL of water was added to the residue, stirred for 30 min, and allowed to stand to separate layers to obtain 12 g of the product with a yield of 87%.
[0021] Preparation of compound 1
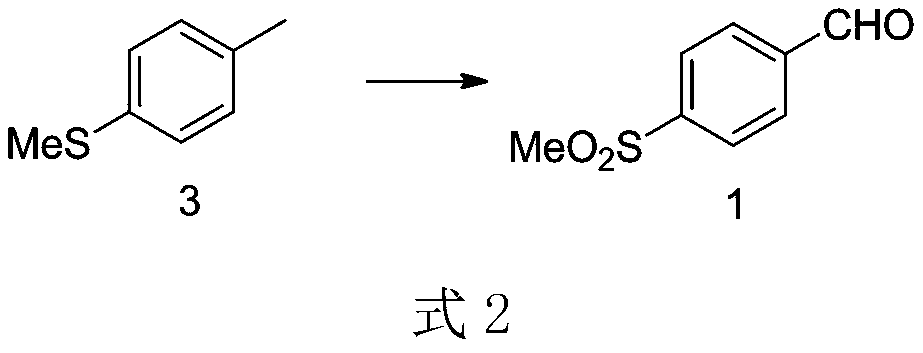
[0022] Air was used as the oxygen source. Mix the mixture of compound 3 (1.4g, 0.01mol) and water with air in a certain proportion and send it into a 220°C preheater, then send the preheated raw material and superheated air into it, which is filled wi...
Embodiment 2
[0023] Embodiment 2: In the synthetic route shown in Formula 1, X is a bromine atom; the catalyst is CuI; the solvent is ethylene glycol dimethyl ether.
[0024] Preparation of compound 3
[0025] Under nitrogen protection, compound 2 (12.6g, 0.1mol) was dissolved in 100mL ethylene glycol dimethyl ether in a 250mL three-necked flask, and 30% sodium methyl mercaptide solution (35.0g, 0.15mol) and a catalytic amount of CuI (0.0003 mol, 0.06g). The reaction mixture was heated to reflux for 8 hours, cooled to room temperature, and the solvent was recovered under reduced pressure. 40 mL of water was added to the residue, stirred for 30 min, and allowed to stand for separation to obtain 11 g of the product with a yield of 81%.
[0026] Preparation of Compound 1
[0027] Air was used as the oxygen source. Mix the mixture of compound 3 (1.4g, 0.01mol) and water with air in a certain proportion and send it into a 220°C preheater, then send the preheated raw material and superheated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com