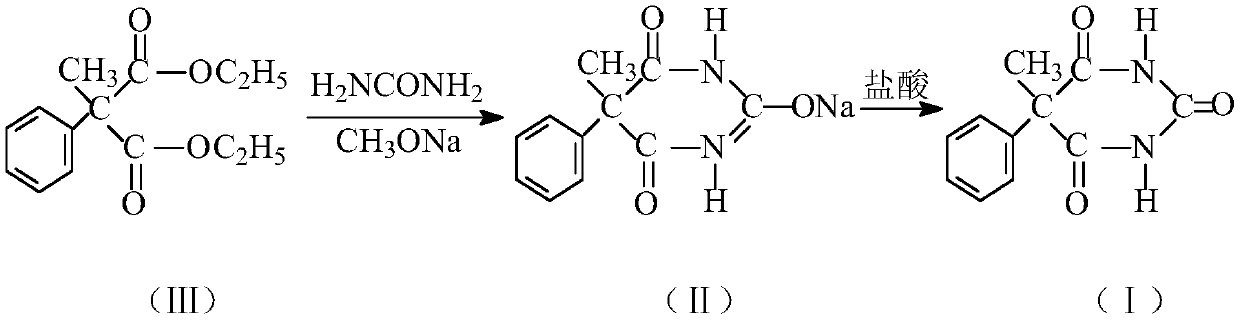
Preparation method of 5-methyl-5-phenylbarbituric acid
A technology of phenylbarbituric acid and diethyl phenylmalonate, applied in the field of preparation of 5-methyl-5-phenylbarbituric acid, can solve the problem of difficult industrial production, high content requirements, Low product yield and other problems, to achieve the effect of stable product quality, high yield and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Step A: Add 313.5 g of sodium methoxide (sodium methoxide content: 31% w / w) methanol solution into the reaction flask, then add 3.5 g of ethyl acetate, heat up to slight reflux for 25 minutes to eliminate free alkali. Then, 108.0 g of urea and 250.3 g of diethyl α-methyl-α-phenylmalonate were added, and the temperature was raised to distill and recover the solvent to an internal temperature of 115°C. The concentrate was decompressed into foam, cooled to below 30°C, added 876g of cold water at 5°C and stirred to dissolve. Then add refined and recovered activated carbon, filter, and the filtrate is acidified with hydrochloric acid until crystallization pH=3.5, filtered, washed with water, and dried to obtain 208.2 g of crude compound (I), with an HPLC content of 99.32%.
[0017] Step B: Add the crude product of compound (I) obtained in step A into the reaction flask, then add 728.7 g of 70% ethanol aqueous solution and activated carbon, heat up and reflux for decolorizati...
Embodiment 2
[0019] Step A: Add 409.7g of sodium methoxide (sodium methoxide content: 29% w / w) methanol solution into the reaction flask, then add 4.5g of ethyl acetate, and heat up to slight reflux for 30 minutes to eliminate free alkali. Then, 108.0 g of urea and 250.3 g of diethyl α-methyl-α-phenylmalonate were added, and the temperature was raised to distill and recover the solvent to an internal temperature of 113°C. The concentrate was decompressed into foam, cooled to below 30°C, added 1001g of cold water at 2°C and stirred to dissolve. Then add refined and recovered activated carbon, filter, and the filtrate is acidified with hydrochloric acid until crystallization pH=4, filtered, washed with water, and dried to obtain 208.6 g of crude compound (I), with an HPLC content of 99.15%.
[0020] Step B: Add the crude product of compound (I) obtained in step A into the reaction flask, then add 834.4 g of 60% ethanol aqueous solution and activated carbon, heat up and reflux for decolorizat...
Embodiment 3
[0021] Example 3: Step A: Add 396g of sodium methoxide (sodium methoxide content is 30% w / w) methanol solution into the reaction flask, then add 4.0g of ethyl acetate, heat up to slight reflux reaction for 20min to eliminate free alkali. Then add 150.0 g of urea, and then add 250.3 g of diethyl α-methyl-α-phenylmalonate, heat up and distill to recover the solvent to an internal temperature of 110°C. The concentrate was decompressed into foam, cooled to below 30°C, and 1126g of cold water at 3°C was added to dissolve it. Then add refined and recovered activated carbon, filter, and the filtrate is acidified by hydrochloric acid to crystallization pH = 4.5, filtered, washed with water, and dried to obtain 210.6 g of crude compound (I), with an HPLC content of 99.29%.
[0022] Step B: Add the crude product of compound (I) obtained in step A into the reaction flask, then add 947.7 g of 50% ethanol aqueous solution and activated carbon, heat up and reflux for decolorization for 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com