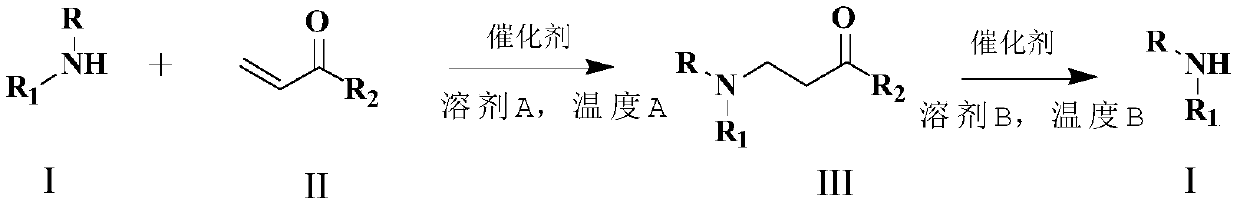
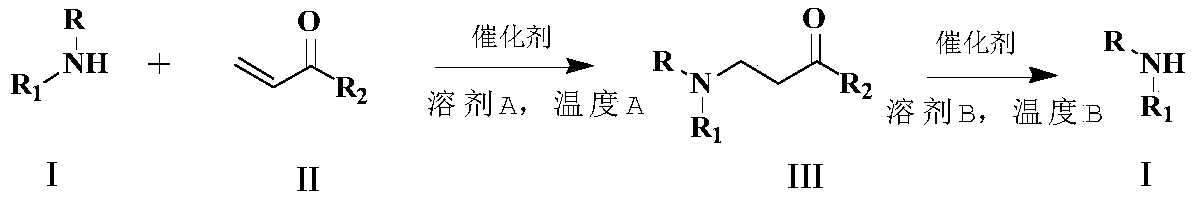
Method for catalyzing amino protection by imidazole hydrochloride
An imidazole hydrochloride catalyzed ammonia and amino protection technology, applied in chemical instruments and methods, preparation of amino compounds, preparation of organic compounds, etc., can solve problems such as toxicity, corrosion, high cost, etc., and achieve good functional group tolerance , the effect of short response time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 General Method for Michael Addition to Occur (1a-1t)
[0052] Amine (1 equivalent), imidazole hydrochloride (0.3 equivalent) and N,N-dimethylacetamide 5 mL were added to a 50 mL three-neck round bottle. Warm the resulting solution to 120°C and stir the reaction at this temperature. TLC plate detection, when the reaction is complete, cool to room temperature, add ethyl acetate (30ml), then add activated carbon for decolorization, filter, and wash with saturated brine organic layer. The organic layer was dried over anhydrous sodium sulfate, filtered, the solvent was removed under reduced pressure, and purified by silica gel column to obtain the target product.
[0053] General method for deprotection (2a-2f)
[0054] Into a 50 mL three-neck round bottle were added Michael addition product (1 equivalent), imidazole hydrochloride (0.7 amount). The resulting mixture was heated to 150°C and stirred at this temperature for reaction, detected by TLC spot plate, whe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com