Preparation method of (3-amino-azetidine-1-yl)-cyclopropyl-ketone
An azetidine and cyclopropyl technology, which is applied in the fields of fine chemical industry and pharmaceutical intermediate synthesis, can solve the problems of being unsuitable for large-scale production, using dangerous reagents, and high process costs, achieving low price and shortening reaction steps. , the effect of improving the reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] A preparation method of (3-amino-azetidin-1-yl)-cyclopropyl-methanone, comprising the following steps:
[0041] (1) Under alkaline conditions, dissolve azetidin-3-one hydrochloride in a solvent, add an acylating reagent to react, and obtain 1-cyclopropanecarbonyl-azetidin-3-one, The reaction process is:
[0042] ;
[0043] Among them, R= or .
[0044] (2) Dissolve 1-cyclopropanecarbonyl-azetidin-3-one in a solvent, add benzylamine, stir to dissolve, add a reducing agent, and react at -5°C~5°C to obtain (3-benzylamine Amino-azetidin-1-yl)-cyclopropyl-methanone, the reaction process is:
[0045] ;
[0046] (3) Dissolve 3-borylamino-azetidin-1-yl)-cyclopropyl-methanone in a solvent, and react with hydrogen in the presence of a catalyst to obtain (3-amino-nitrogen Heterobutan-1-yl)-cyclopropyl-methanone, the reaction process is:
[0047] .
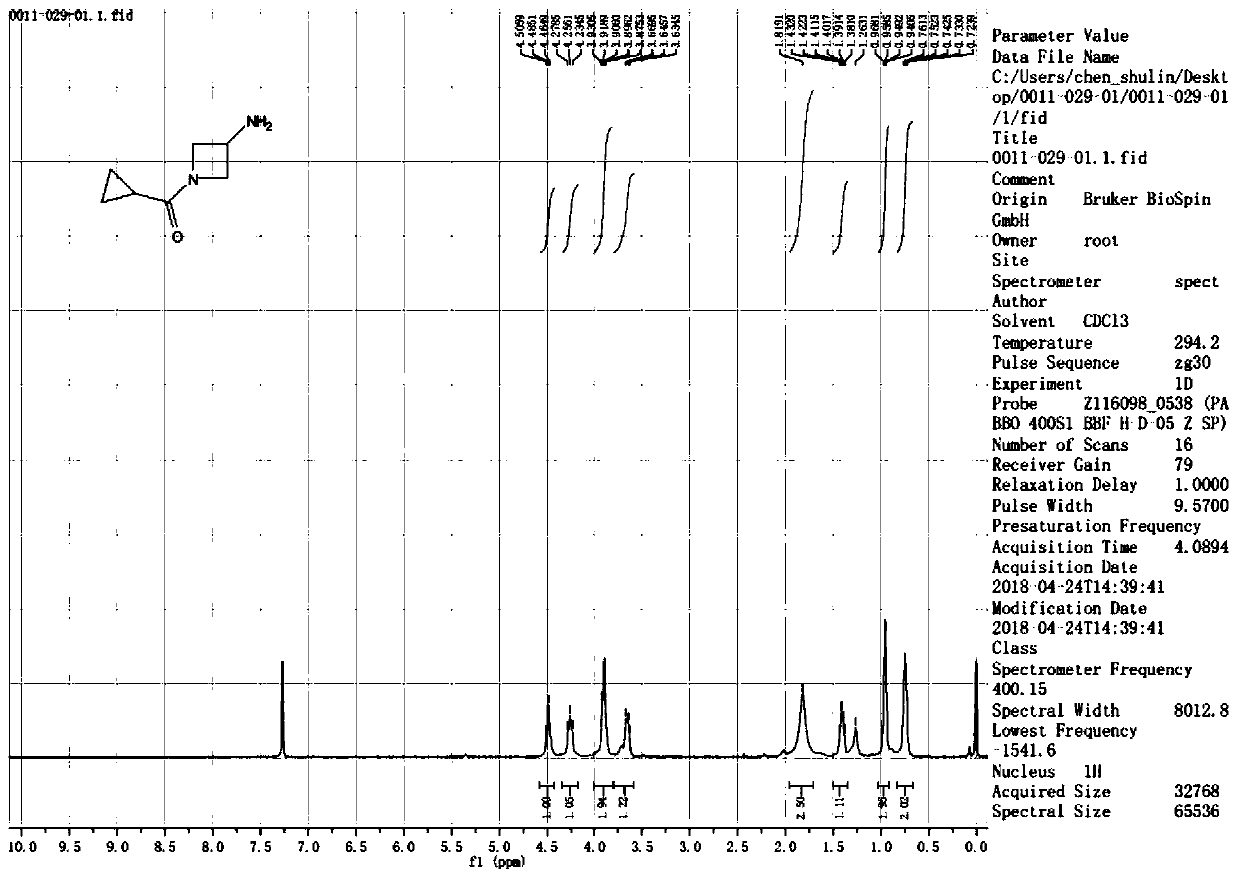
[0048] (3-amino-azetidin-1-yl)-cyclopropyl-methanone obtained by the present invention 1 HNMR picture as shown figu...
Embodiment 1
[0050] (1) Synthesis of 1-cyclopropanecarbonyl-azetidin-3-one:
[0051]
[0052] Add azetidin-3-one hydrochloride (32.30 g, 0.30 mol, 1.0 equiv.) and triethylamine (75.89 g, 0.75 mmol, 2.5 equiv.) into 300 ml of dichloromethane, stir at room temperature After 30 minutes, cyclopropanoyl chloride (34.49 g, 0.33 mol, 1.1 equiv.) was added dropwise, and the mixture was reacted at room temperature for 3 hours. TLC showed that the reaction was complete. Add saturated Na2 CO 3 The solution was 100 ml, the organic phase was separated, the aqueous phase was extracted twice with dichloromethane, and the organic phases were combined. The organic phase was washed twice with 1 N aqueous hydrochloric acid solution, once with saturated brine, and washed with anhydrous Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to obtain 35.90 g of a light yellow solid, with a yield of 86%.
[0053] LC-MS (ESI+): m / z 140.16 (M+H).
[0054] (2) Synthesis of (3-benzylamino-azetidin-1-...
Embodiment 2
[0064] (1) Synthesis of 1-cyclopropanecarbonyl-azetidin-3-one:
[0065]
[0066] At room temperature, cyclopropanecarboxylic acid (2.07 g, 24 mmol, 1.20 equiv.), azetidin-3-one hydrochloride (2.15 g, 20 mmol, 1.0 equiv.), 1-ethyl-(3 -Dimethylaminopropyl)carbodiimide hydrochloride (EDCI) (8.43 g, 44 mmol, 2.2 equiv.) and 4-dimethylaminopyridine (DMAP) (4.89 g, 40 mmol, 2.0 equiv.) Added successively to 40 ml of acetonitrile, stirred overnight at room temperature, TLC showed that the reaction was complete. Add 100 ml of water and extract with ethyl acetate (100 ml*3). The organic phases were combined, and the organic phase was once washed with 1N hydrochloric acid, saturated NaHCO 3 , washed with saturated brine, anhydrous Na 2 SO 4 Dry, filter, and concentrate under reduced pressure to obtain 2.09 g of a light yellow solid, with a yield of 75%.
[0067] LC-MS (ESI+): m / z 140.16 (M+H).
[0068] (2) Synthesis of (3-benzylamino-azetidin-1-yl)-cyclopropyl-methanone:
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com