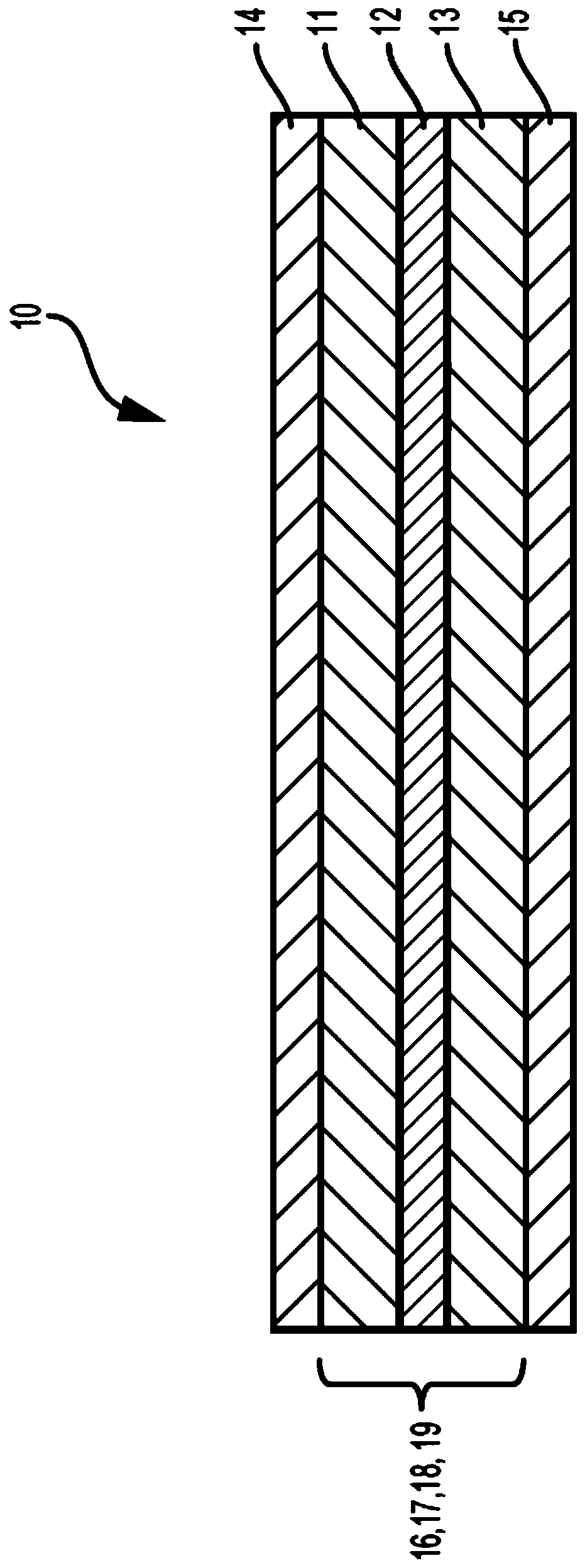
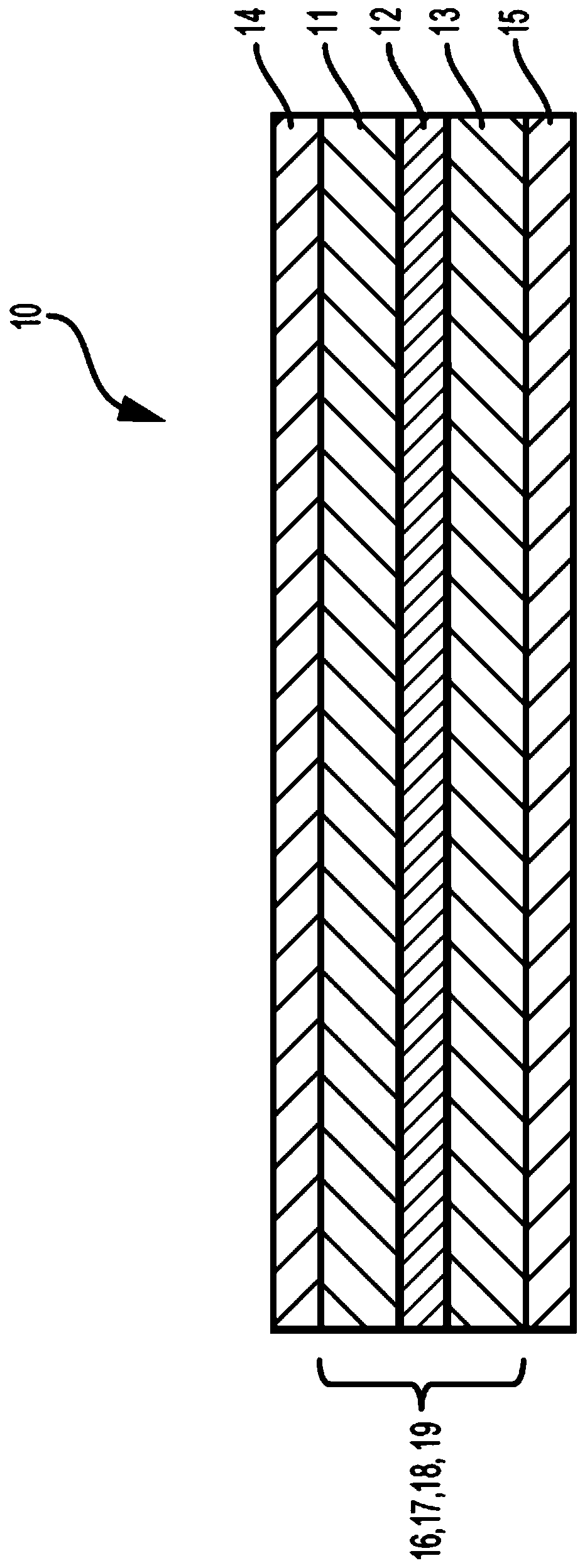
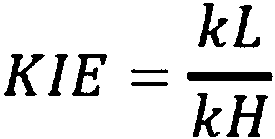Deuterated electrolyte solvents
An electrolyte and organic solvent technology used in the field of rechargeable batteries
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0063] The following reactions show the production of methane from DMC in the overdischarged state:
[0064] CH 3 OCO 2 CH 3 +2e - +2Li + →2CH3 OLi↓+CO↑
[0065] CH 3 OCO 2 CH 3 +2e - +2Li + h 2 → Li 2 CO 3 ↓+2CH 4
[0066] CH 3 OCO 2 CH 3 +e - +Li + 1 / 2H 2 →CH 3 OCO 2 Li↓+CH 4 ↑
[0067] In an ideal alternative, the same mechanism involving deuterium has a much slower reaction rate:
[0068] cd 3 OCO 2 cd 3 +2e - +2Li + →2CD 3 OLi↓+CO↑
[0069] cd 3 OCO 2 cd 3 +2e - +2Li + D. 2 → Li 2 CO 3 ↓+2CD 4
[0070] cd 3 OCO 2 cd 3 +e - +Li + 1 / 2D 2 →CD 3 OCO 2 Li↓+CD 4 ↑
[0071] The use of DEC and EMC involves reactions similar to those shown above.
example 2
[0073] Consider the following reactions that occur in an overcharged state:
[0074] 3CO 2 →Co 3 o 4 +O 2 ↑
[0075] CH 3 OCO 2 CH 3 (DMC)+3O 2 →3CO 2 ↑+3H 2 o
[0076] In an ideal substitution situation, the same mechanism would have slower reaction kinetics:
[0077] 3CO 2 →Co 3 o 4 +O 2 ↑
[0078] cd 3 OCO 2 cd 3 (DMC)+3O 2 →3CO 2 ↑+3D 2 o
[0079] The final reactions show, respectively, the generation of oxygen due to the degradation of the cathode material and the decomposition of the electrolyte due to the generated oxygen. It should be noted that in this case the formation of water would be an obstacle, since it would be more difficult to "extract" the deuterium from the solvent. Furthermore, water is particularly detrimental to cell stability because it is a precursor to hydrofluoric acid.
example 3
[0081] Electrolyte preparation
[0082] Lithium salts (e.g., LiPF 6 ) added to ethylene carbonate-d 4 , diethyl carbonate-d 10 and dimethyl carbonate-d 6 , so that a certain molar concentration of electrolyte is obtained. In other examples, lithium salts are added to ethylene carbonate-d 4 / Ethylene carbonate, diethyl carbonate-d 10 / Diethyl carbonate and dimethyl carbonate-d 6 / dimethyl carbonate, the ratio of isotopologue to organic solvent in said mixture is 1:10 points and 1:50. Also, for testing purposes, known concentrations of contaminants such as water and hydrofluoric acid are added to the electrolyte using a moisture titrator. In other examples, ethylene carbonate-d 4 , diethyl carbonate-d 10 and dimethyl carbonate-d 6 Other isotopic substitutions are included, such as carbon-13 in place of carbon-12.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com