Method for calculating and determining absolute configuration of chiral amines according to nuclear magnetic resonance fluorine spectrum theory
A theoretical calculation and absolute configuration technology, applied in molecular entity identification, analysis of two-dimensional or three-dimensional molecular structure, instruments, etc., can solve problems such as inconsistency of facts and deviation of configuration determination results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
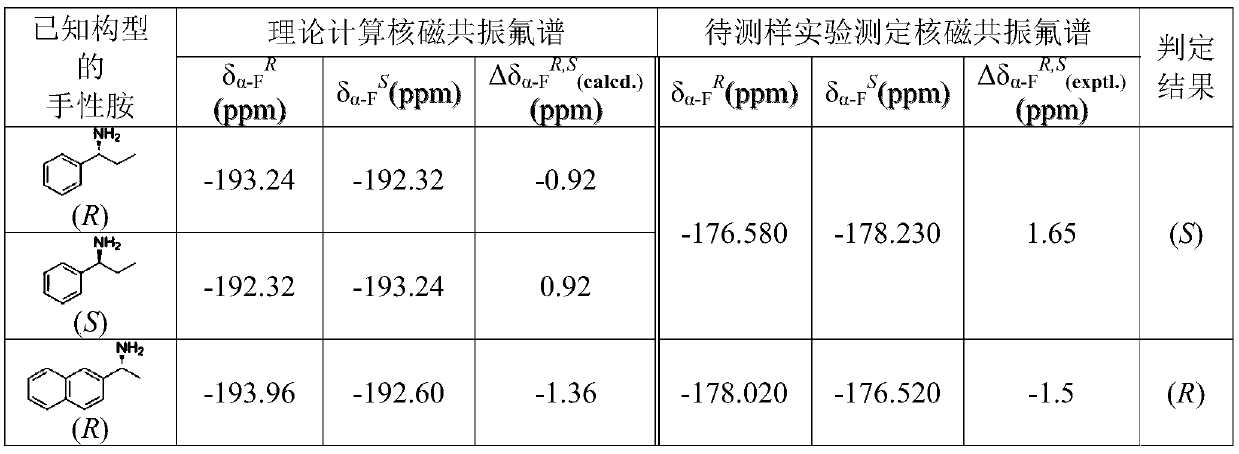
[0012] Example 1 Determination of the Absolute Configuration of Optical α-Phenylethylamine
[0013] The specific implementation method of determining the absolute configuration by the nuclear magnetic resonance fluorine spectrum theoretical calculation method is as follows:
[0014] i) The molecular structural formula of the amidation product generated by the reaction of (R)-α-fluorophenylacetate and (S)-α-phenylethylamine was initially simulated by the ball and stick model in the Gaussian 09 system. Optimization, to obtain the lowest Gibbs free energy conformer, obtain the calculated value of the chemical shift of the α-fluorine of the derivative δ α-F R (calcd.) =-192.32ppm.
[0015] ii) The molecular structural formula of the amidation product generated by the reaction of (S)-α-selenofluorophenylacetate and (S)-α-phenylethylamine was initially simulated by the ball-and-stick model in the Gaussian 09 system. Optimization, to obtain the lowest Gibbs free energy conformer,...
example 2
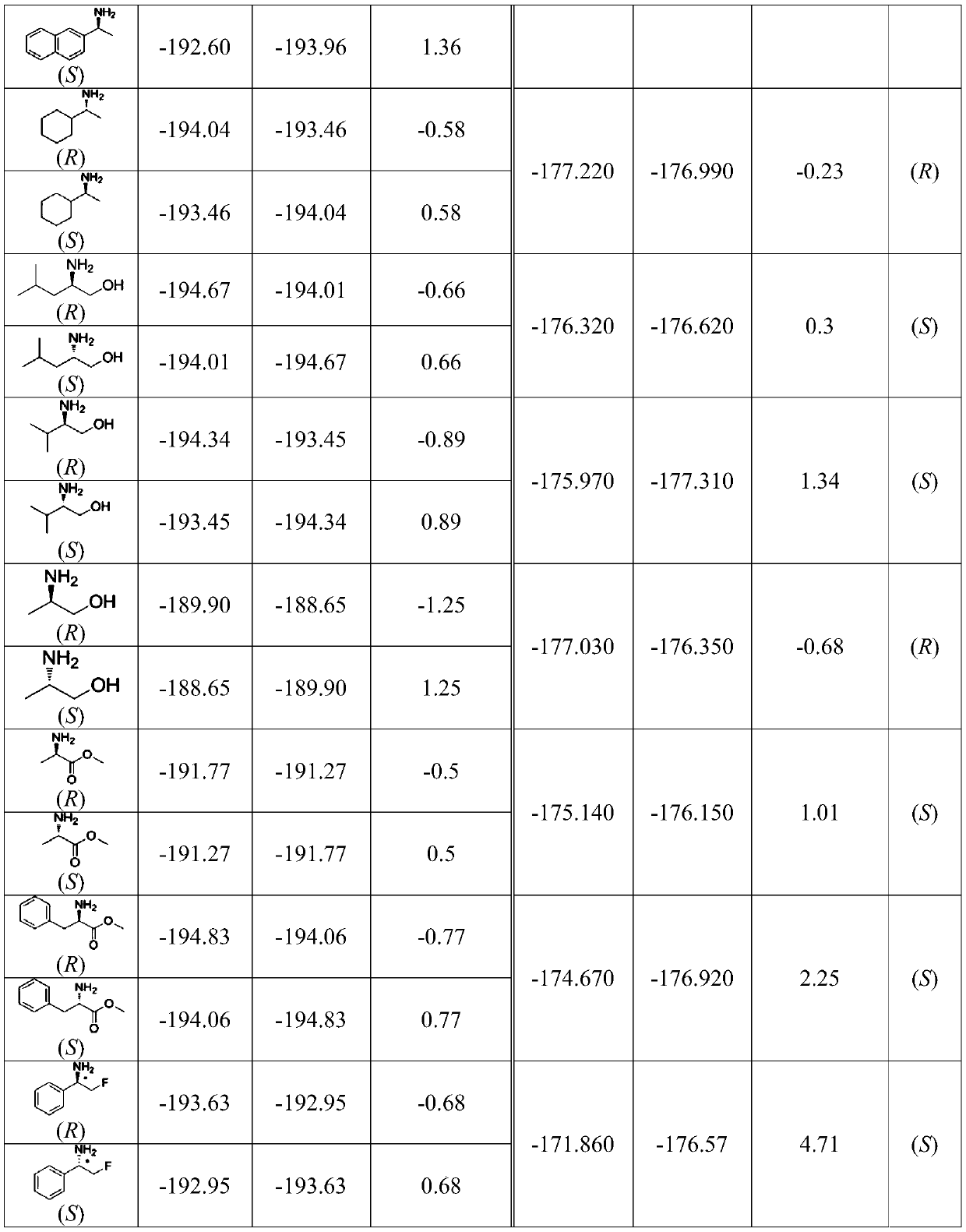
[0019] Example 2 Determination of the Absolute Configuration of Optical Amphetamine
[0020] The specific implementation method of determining the absolute configuration by the nuclear magnetic resonance fluorine spectrum theoretical calculation method is as follows:
[0021] i) The molecular structure of the amidation product produced by the reaction of (R)-α-selenofluorophenylacetate and (R)-2-amino-3-phenyl-1-propanol was performed on the ball-and-stick model in the Gaussian 09 system Preliminary simulation of , through continuous geometric optimization, the lowest Gibbs free energy conformer is obtained, and the calculated value of the chemical shift of the derivative α-fluorine δ α-F R (calcd.) =-191.64ppm.
[0022] ii) The molecular structure of the amidation product produced by the reaction of (S)-α-selenofluorophenylacetate and (R)-2-amino-3-phenyl-1-propanol was performed on a Gaussian 09 system using a ball-and-stick model Preliminary simulation of , through cont...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com