Recombinant cellulase with primary product cellotetrose and building method and application thereof
A cellotetraose and cellulase technology, applied in the field of enzyme engineering, can solve the problems of high production cost and complicated purification process, and achieve the effects of high concentration, high purity and high enzyme activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Obtaining the cellulase that continuously produces and accumulates cellotetraose includes the following steps:
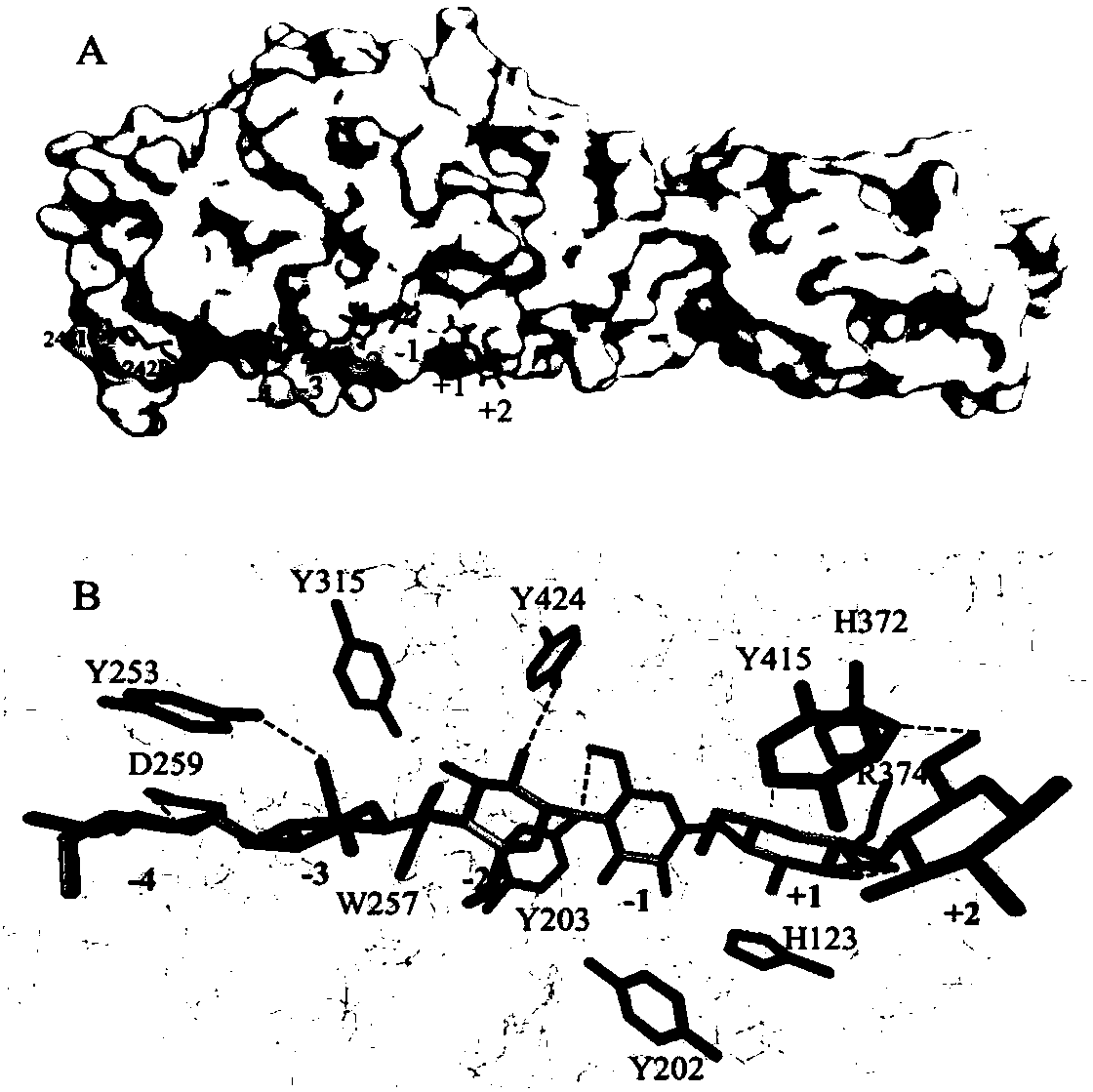
[0047] 1) Homology modeling and molecular docking: Using the three-dimensional structures of Cel9G (PDB: 1GA2) in Clostridium cellulolyticum and Cel9A (PDB: 4TF4) in Thermobifida fusca as templates, use Discovery Studio 4.0 Construct the structural model of the target sequence; then use Pymol 1.7 (http: / / www.pymol.org) to use the cellulose single chain (the cellulose single chain is a cellulose chain greater than 6 glucose units, which can fill the active center of the model) Molecular docking was carried out between the cellulose single chain and the structure model of the above obtained enzyme molecule, and the enzyme with the same structural domain as CcCel9A was obtained.
[0048] And in the obtained model, the active center of the enzyme molecule and the amino acid site where the CBM region binds to the substrate are marked.
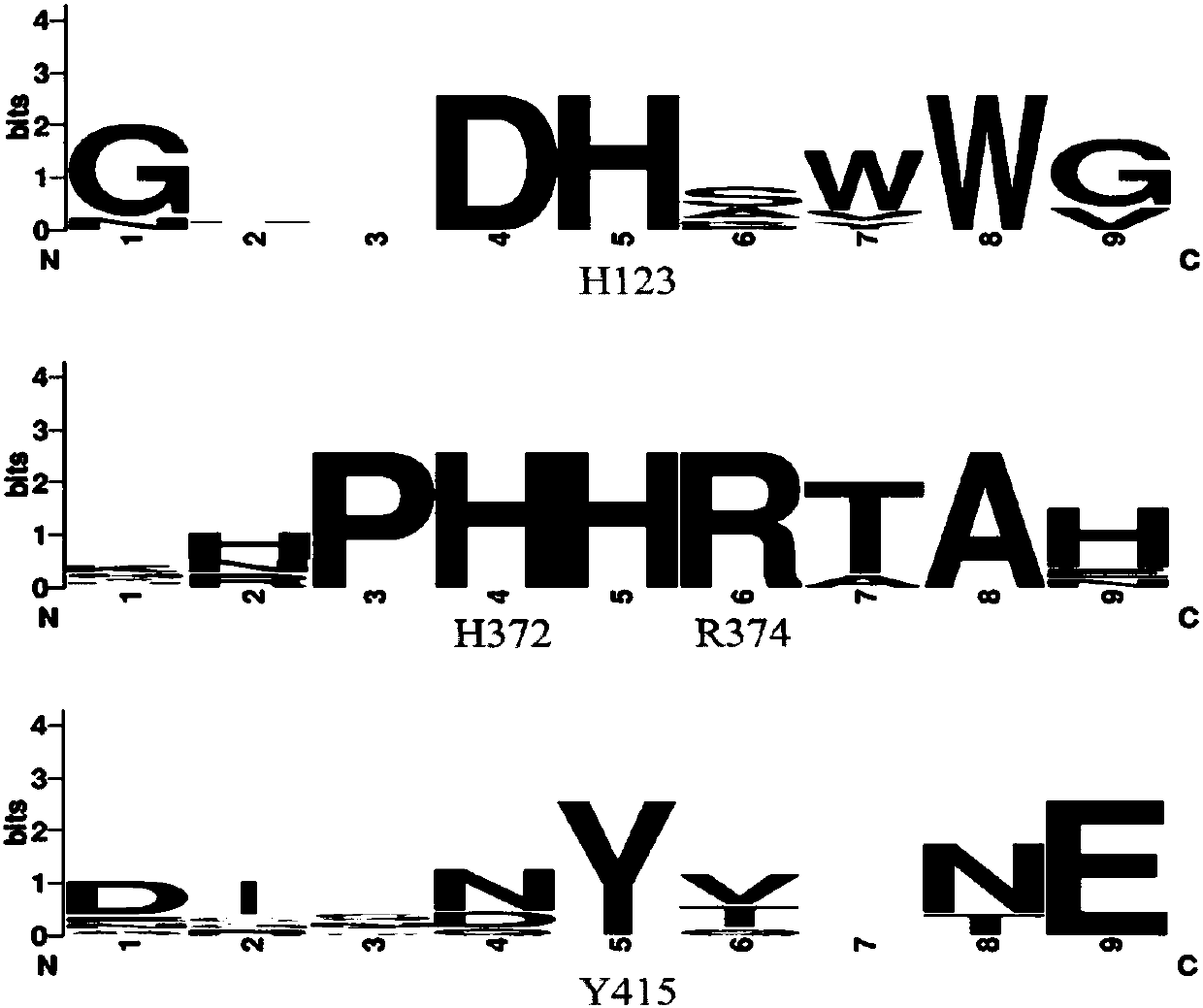
[0049] 2) Selection of mutat...
Embodiment 2
[0107] Example 2: Enzymatic hydrolysis of amorphous cellulose by point mutants
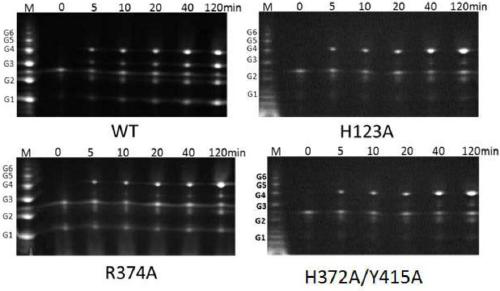
[0108] After expressing and purifying the point mutant protein obtained from the above construction, it was added to the cellulose substrate for the production of cellotetraose, and the wild type was also used as a control. The reaction system was as follows: Add to 10g / L amorphous cellulose Enzyme with a final concentration of 1 μM and a total volume of 5 mL were incubated at 60° C. for 48 hours. After the protein was inactivated, the supernatant was collected by centrifugation for detection of product spectrum.
[0109] Among them, the product map analysis of point mutants: add 3 μM of the above-mentioned purified enzyme solution obtained with different point mutations to 50 μl of 1% RAC solution, then add PC buffer to a total volume of 100 μl, and place at 60° C. for reaction. Samples were taken at 5 minutes, 10 minutes, 20 minutes, 40 minutes, and 120 minutes, and the volume of each sample was...
Embodiment 3
[0112] Example 3: Point mutant proteins participate in the compounding of cellulase preparations
[0113]After expressing and purifying the constructed point mutant protein H372A / Y415A, it was compounded with Novozymes cellulase preparation Cellic CTec2, and mixed in 10g / L corn stover after gas explosion treatment at a molar ratio of 1:5 Add the above compounded enzyme at a final concentration of 5 μM to a total volume of 5 mL, and incubate at 60° C. for 48 h. Take the sample without point mutant protein H372A / R374A as a control, and take the same treatment as the experimental group. After inactivating the protein, the supernatant was centrifuged to detect the product. The results showed that the yields of cellooligosaccharides in the experimental group were 30% and 40% respectively compared with the control group. Sugar production increased by 10%. It can be seen that mixing the enzymes obtained in the above examples with existing enzymes for degradation can produce a syner...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com