Clerodane diterpenoid compounds and application thereof in pharmacy
A technology of crotane-type diterpenes and compounds, which is applied in the directions of organic chemistry, drug combination, and pharmaceutical formulations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
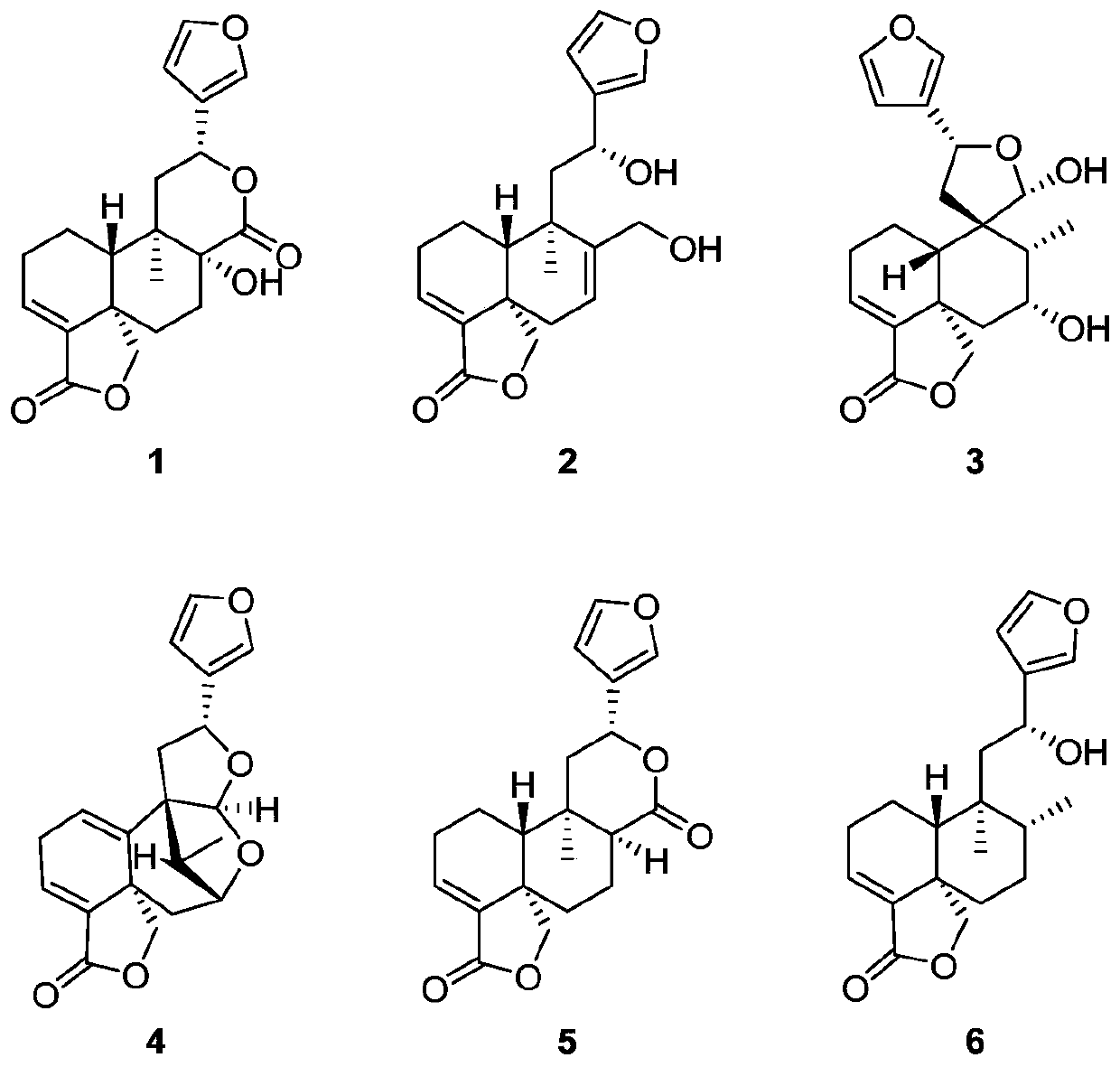
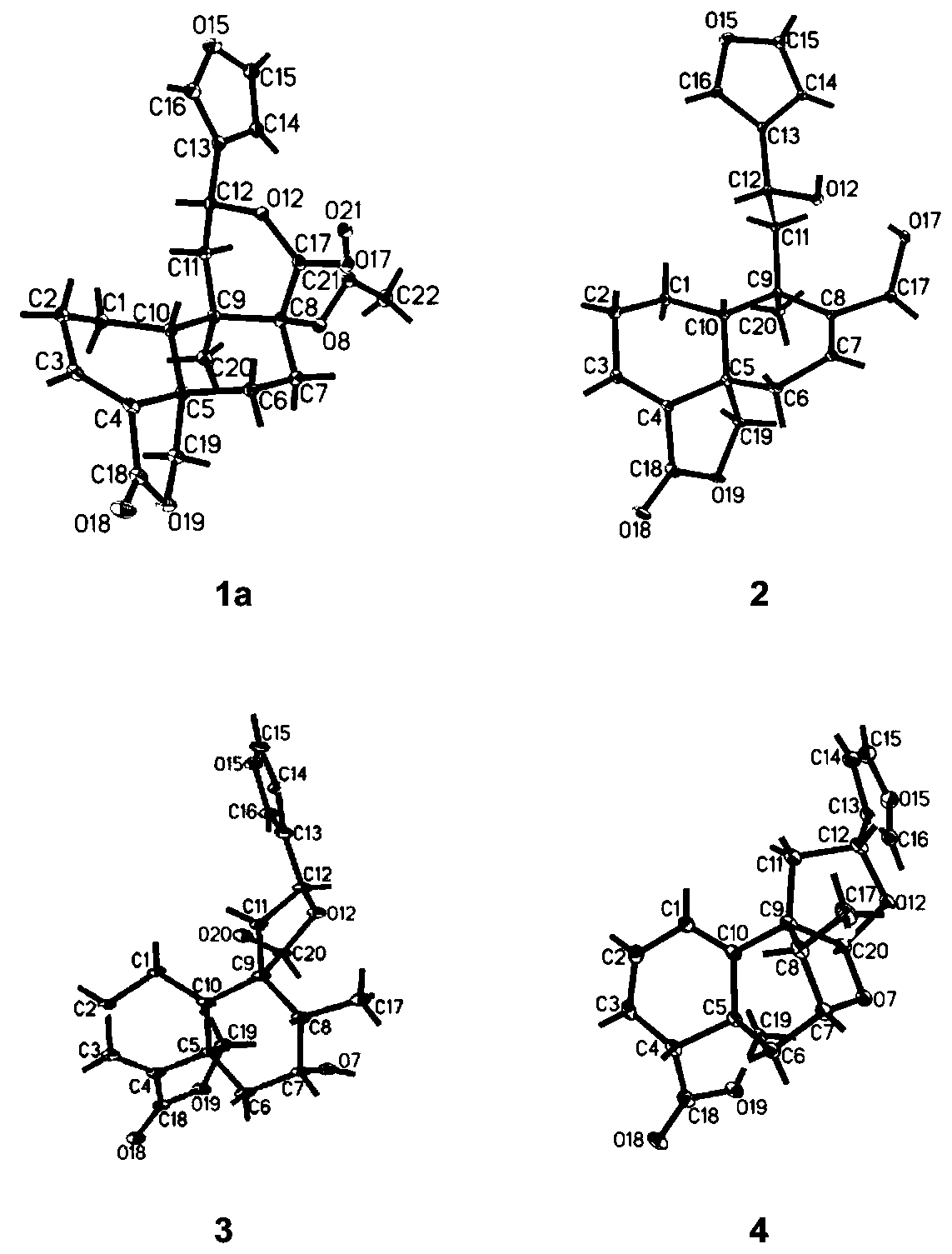
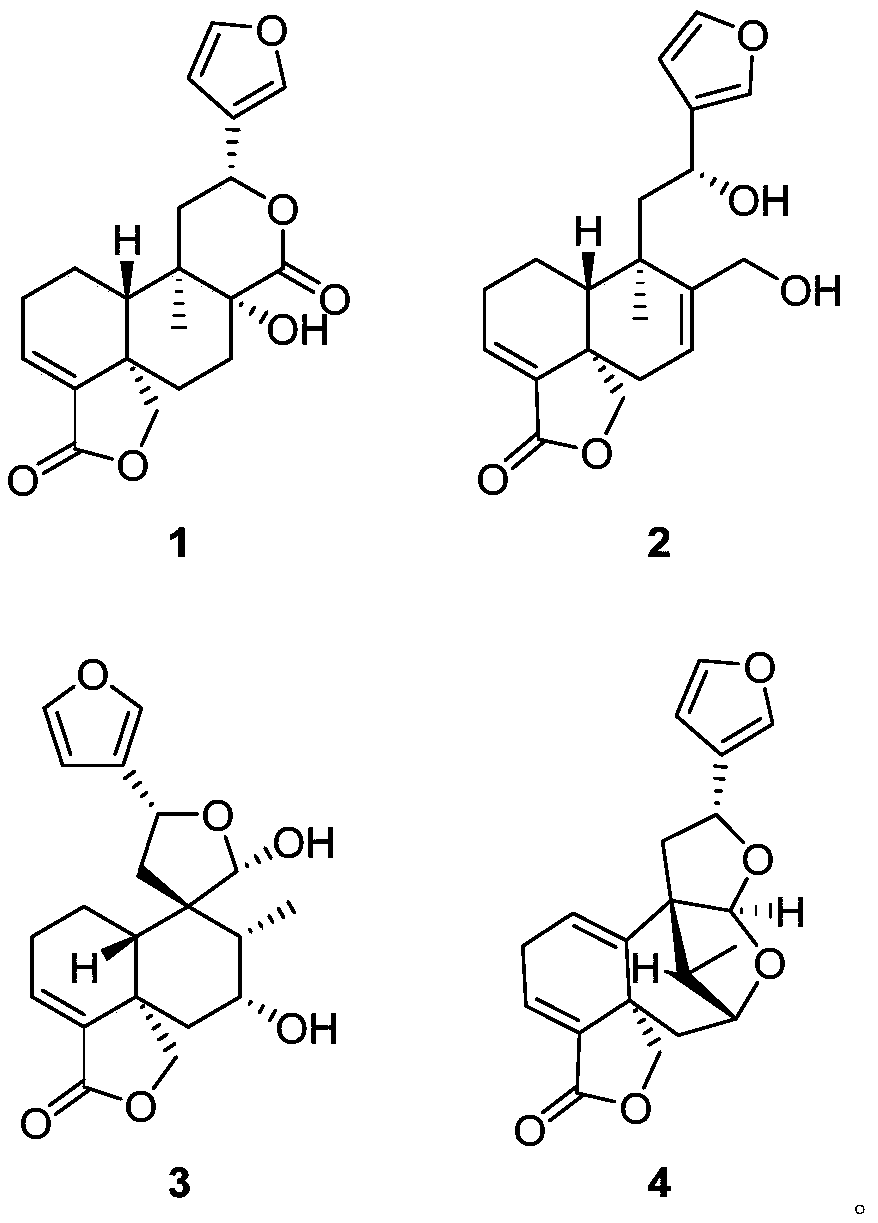
[0032] The preparation method and structural identification of Crone-type diterpene compound 1-6:
[0033] Preparation method: Take the aboveground part of Salvia hispanica, dry it and crush it, soak it in acetone for three times and extract it three times, combine the three extracts, concentrate under reduced pressure to obtain the total extract, which is subjected to silica gel column chromatography , the elution system is petroleum ether / acetone, detected by TLC, combined according to the main spots, and 5 components Fr.1-Fr.5 are obtained, and then Fr.3 is mixed with polyamide, dried and packed into a column. Select MCI reverse-phase chromatography column, connect to medium-pressure liquid chromatograph, select ethanol / water gradient elution, after vacuum distillation and concentration, each fraction is detected by TLC and merged according to the main spots to obtain 8 groups of Fr .3.1-Fr.3.8, Fr.3.1 was subjected to silica gel column chromatography, and the elution syste...
Embodiment 2
[0053] Evaluation of the protective effect of compounds 1-6 on hydrogen peroxide-induced injury in primary neonatal rat cardiomyocytes:
[0054] 1. Experimental method
[0055] 1.1 Culture of primary cardiomyocytes in suckling mice
[0056] Select newborn SD suckling mice 1-3 days old, disinfect the skin with 75% alcohol, decapitate, open the chest and take out the heart. Drain the blood from the heart and cut off excess tissue, cut it into 1-2mm 3 sized tissue pieces and transferred to a 15 mL centrifuge tube. Discard the DMEM high-glucose medium, add digestion solution, and repeatedly blow and digest with a dropper until the tissue pieces are completely digested. The culture solution in each centrifuge tube was filtered through a 200-mesh filter, repackaged, and centrifuged at 1000r / min for 5min. Discard the supernatant, add new cell culture medium, pipette repeatedly with a dropper to disperse into single cells, and dilute with 1×10 5 cells / mL were inoculated into a 96...
Embodiment 3
[0073] Preparation of tablets:
[0074] According to Example 1, various compounds of the present invention were firstly prepared, and the excipients were added in a weight ratio of 1:5-1:10, granulated and pressed into tablets according to their own independence or arbitrary mixing.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com