Method for preparing anhydrous magnesium carbonate based on hydrothermal carbonization reaction
An anhydrous magnesium carbonate and hydrothermal carbonization technology, applied in magnesium carbonate and other directions, can solve the problems of harsh reaction environment, large crystal particle size and long reaction time, and achieve the effects of uniform particle size, good dispersion and simple equipment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
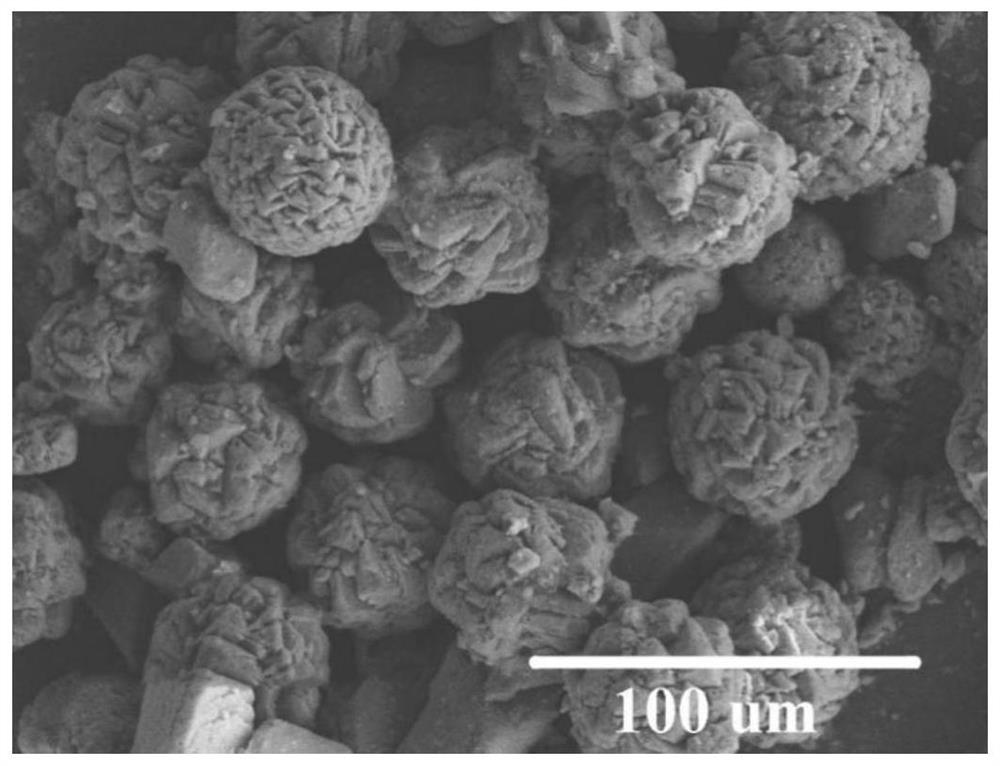
[0025] Adjust the initial pH of 150mg / ml ascorbic acid aqueous solution to 8.5, add MgCl 2 As a magnesium source, control Mg 2+ The concentration was 1.0 mol / L. The above mixture was magnetically stirred for 20 minutes, then placed in a 50ml reaction kettle, and then the reaction kettle was put into an oven, and the temperature of the oven was set at 180°C. After 3 hours of reaction, the reaction kettle was naturally cooled to room temperature. The reacted mixture was centrifuged (4000rpm, 5min), the solid product was washed three times with ethanol, and dried in an oven (80°C, 12h) to obtain anhydrous magnesium carbonate powder. Depend on figure 1 It can be seen that the obtained anhydrous magnesium carbonate is a sphere with a rough surface and a diameter of about 5um.
Embodiment 2
[0027] Adjust the initial pH of 150mg / ml ascorbic acid aqueous solution to 13.5, add MgCl 2 As a magnesium source, control Mg 2+ The concentration was 1.0 mol / L. The above mixture was magnetically stirred for 20 minutes, then placed in a 50ml reaction kettle, and then the reaction kettle was put into an oven, and the temperature of the oven was set at 180°C. After 3 hours of reaction, the reaction kettle was naturally cooled to room temperature. The reacted mixture was centrifuged (4000rpm, 5min), the solid product was washed three times with ethanol, and dried in an oven (80°C, 12h) to obtain anhydrous magnesium carbonate powder. Depend on figure 2 It can be seen that the obtained anhydrous magnesium carbonate is in the shape of a hydrangea with a diameter of about 0.5-1um.
Embodiment 3
[0029] Adjust the initial pH of 150 mg / ml ascorbic acid aqueous solution to 12.5, add MgCl 2 As a magnesium source, control Mg 2+ The concentration was 2.2mol / L, the above mixture was magnetically stirred for 20min, then placed in a 50ml reaction kettle, and then the reaction kettle was put into an oven, the temperature of the oven was set at 180°C, and the reaction kettle was naturally cooled to room temperature after 3 hours of reaction. The reacted mixture was centrifuged (4000rpm, 5min), the solid product was washed three times with ethanol, and dried in an oven (80°C, 12h) to obtain anhydrous magnesium carbonate powder. Depend on image 3 It can be seen that the obtained anhydrous magnesium carbonate is in the shape of a jagged rod.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com