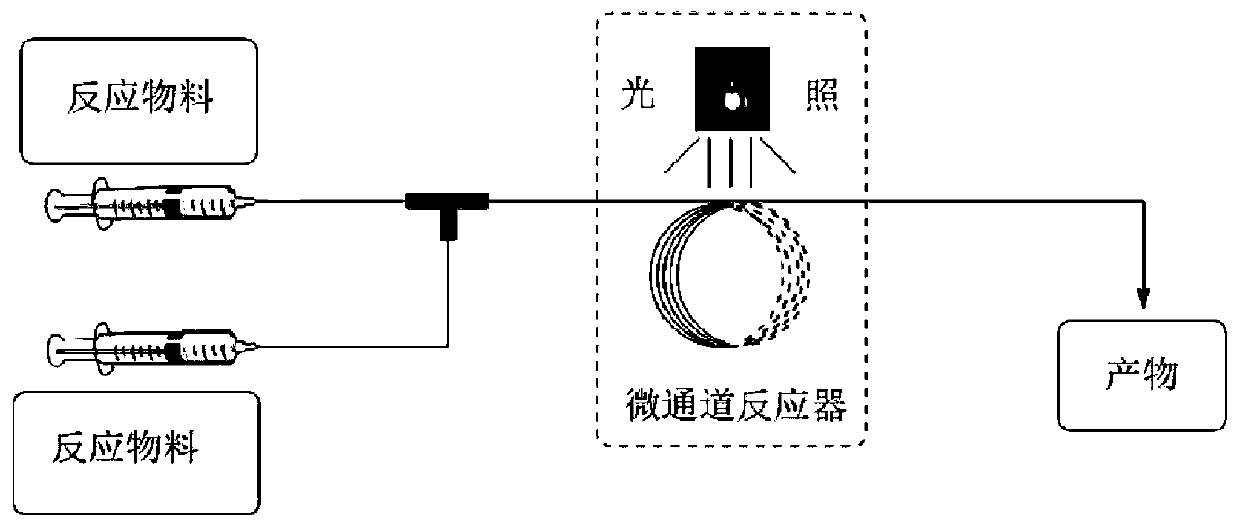
Method for preparing 3,4-dihydroisoquinoline-2(1H)-one compound through photocatalytic micro-channel
A ketone compound, dihydroquinoline technology, applied in chemical instruments and methods, chemical/physical processes, chemical/physical/physical chemical processes, etc., can solve expensive fluorine sources and transition metal catalysts, many synthesis steps, environmental problems Unfriendly and other problems, to achieve the effect of improving reaction yield, high reaction yield, and simple construction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] (E)-4-(Chloro(phenyl)methylene)-3-methyl-1-toluenesulfonyl-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinone Synthesis of Lin-2(1H)-ones. Take 83mg of 1a (0.2mmol, 1eq), 10-methyl-9-mesitylylacridine perchlorate (5mol% of 1a) was dissolved in 1mL of acetonitrile, sodium trifluoromethanesulfinate (0.6mmol , 3eq), N-chlorophthalimide (0.24mmol, 1.2eq) was dissolved in 1mL of acetonitrile, the above solutions were added to the syringe and pumped into the microchannel reactor using a syringe pump, the inner diameter of the reactor was 0.5mm, The volume was 1 mL, the reaction residence time was 36 s, irradiated with 50 W blue light with a wavelength of 455 nm, and the temperature was controlled at 25 ° C. After the reaction, post-treatment was performed to obtain 101.8 mg of the final product, with a yield of 98%. The characterization data are as follows: 1 H NMR (500MHz, CDCl 3 )δ7.90(d, J=8.0Hz, 2H), 7.81(d, J=8.0Hz, 2H), 7.54(t, J=7.7Hz, 1H), 7.49–7.42(m, 3H), 7.39– 7.34(m,...
Embodiment 2
[0057] (E)-4-(Chloro(phenyl)methylene)-3-methyl-1-toluenesulfonyl-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinone Synthesis of Lin-2(1H)-ones. Take 83mg of 1a (0.2mmol, 1eq), 10-methyl-9-mesitylylacridine perchlorate (19mol% of 1a) was dissolved in 0.2mL of acetonitrile, sodium trifluoromethylsulfinate (1mmol , 5eq), N-chlorophthalimide (1mmol, 5eq) was dissolved in 3mL of acetonitrile, the above solutions were added to the syringe and pumped into the microchannel reactor using a syringe pump, the inner diameter of the reactor was 1mm, and the length was 1m. The reaction residence time was 60s, irradiated with 60W blue light with a wavelength of 455nm, and the temperature was controlled at 55°C. After the reaction, post-treatment was carried out to obtain 92.5mg of the final product, with a yield of 89%.
Embodiment 3
[0059] (E)-4-(Chloro(phenyl)methylene)-3-methyl-1-toluenesulfonyl-3-(2,2,2-trifluoroethyl)-3,4-dihydroquinone Synthesis of Lin-2(1H)-ones. Take 83mg of 1a (0.2mmol, 1eq), terpyridyl ruthenium dichloride hexahydrate (5mol% of 1a) was dissolved in 1mL of 1,2-dichloroethane, sodium trifluoromethylsulfinate (0.6mmol, 3eq) , tetrabutylammonium chloride (0.24mmol, 1.2eq) was dissolved in 1mL of acetonitrile, the above solutions were added to the syringe and pumped into the microchannel reactor with a syringe pump, the inner diameter of the reactor was 0.5mm, and the volume was 1mL. The residence time was 36s, irradiated with 12W blue light with a wavelength of 455nm, and the temperature was controlled at 25°C. After the reaction, post-treatment was carried out to obtain 100.6 mg of the final product, with a yield of 97%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| strength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com