A kind of berberine anti-tumor platinum (ii) complex and its synthesis method and application
An anti-tumor drug, anti-tumor technology, applied in the direction of anti-tumor drugs, platinum-based organic compounds, platinum-based organic compounds, etc., to achieve the effects of mild reaction conditions, targeted inhibition of proliferation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] In high temperature pressure tube were weighed 1.0mol derivative 1,0.8mol 3.20mol of potassium iodide and dimethyl pyridine amine, 5.0 ml of acetonitrile was added, the solvent was added to dissolve the solid test just completed, more fully reacted, in 90 after 12 hours of reaction at ℃, to give a yellow solid powder B-TFA ligand, a yield of 94.0%.
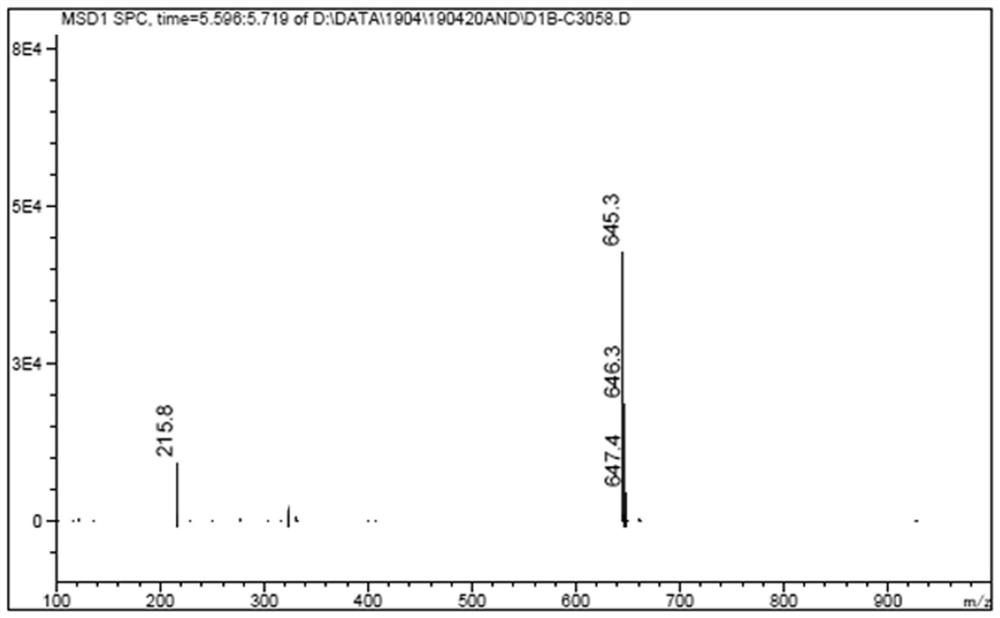
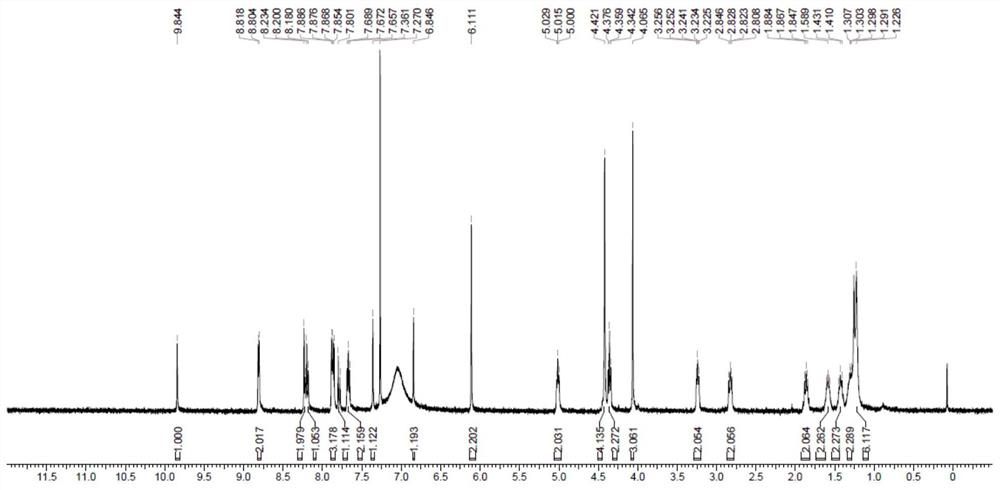
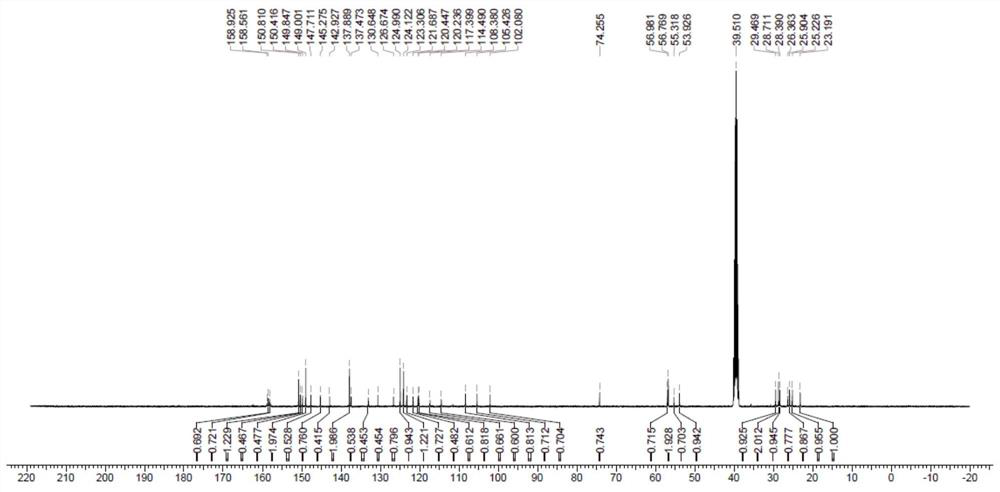
[0036] Weigh ligand B-TFA 1.0mol dichloride and bis (dimethyl sulfoxide) platinum (II) 1.0mol 100.0mL pressure bottle was placed in a high temperature, was added 25.0 mL of acetonitrile, at 60 deg.] C for coordination reaction 4.0h, washed with acetonitrile and dried in vacuo at 45 ℃, i.e. to give the desired product as a yellow Pt1. The yield was 90.8%.
[0037] Berberine present invention antitumor platinum (II) complex scheme is as follows:
[0038]
Embodiment 2
[0040] Unlike Example 1, except that the ligand weighed B-TFA (1.0mol) dichloride and bis (dimethyl sulfoxide) platinum (II) (1.0mol) was placed in a high temperature pressure 100.0mL flask, a solution of 10.0 mL of acetonitrile, is coordinated reaction of 4.0h at 60 deg.] C, washed with acetonitrile and dried in vacuo at 45 ℃, i.e. to give the desired product as a yellow Pt1. The yield was 85.6%.
Embodiment 3
[0042] Unlike Example 1, except that the ligand weighed B-TFA (1.0mol) dichloride and bis (dimethyl sulfoxide) platinum (II) (1.0mol) was placed in a high temperature pressure 100.0mL flask, 75.0 mL of acetonitrile, is coordinated reaction of 4.0h at 60 deg.] C, washed with acetonitrile and dried in vacuo at 45 ℃, i.e. to give the desired product as a yellow Pt1. The yield was 80.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com