Application of 5-methyl-dihydrobenzofuran-imidazole salts in pharmacy
A salt compound, dihydrobenzene technology, applied in the field of medicine, can solve the problems of large toxic and side effects, poor pharmacokinetic properties, anti-tumor and anti-tumor stem cell activity has not been reported, and achieves inhibition of proliferation, induction of apoptosis, The effect of inducing apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
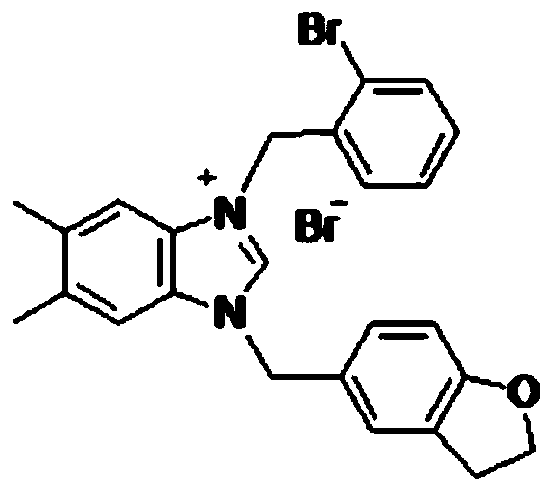
[0062] Preparation of 5-methyl-dihydrobenzofuran-imidazolium salt compounds shown in general structural formula I and II:
[0063] Using 2,3-dihydrobenzofuran as raw material, carry out Vilsmeier reaction to synthesize 5-formyl-dihydrobenzofuran, which is reduced to 5-methanolyl-dihydrobenzofuran with sodium borohydride in alcohol solution Furan, then converted to mesylate, and then reflux reaction with imidazole or substituted benzimidazole in a solvent to synthesize 5-methyl-dihydrobenzofuran-imidazole, and on this basis, react with halides to synthesize 5-methyl Base-dihydrobenzofuran-imidazolium salt compounds.
Embodiment 2
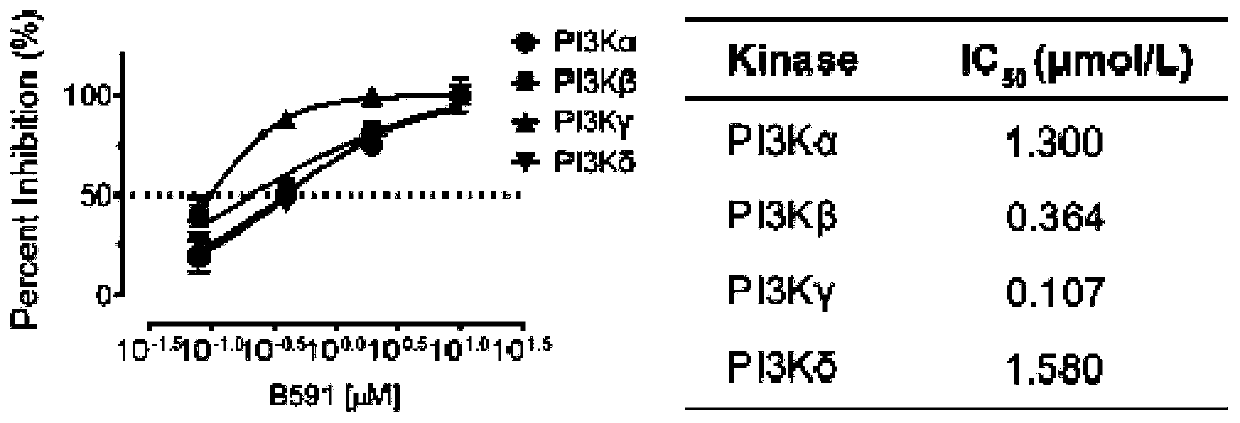
[0065] B591 is a PI3K kinase inhibitor.
[0066] 1. Experimental method: PI3K activity detection kit (purchased from Echelon) was used to detect the effect of B591 (see Figure 1A for the structural formula) on PI3K kinase activity according to the operating instructions. PIP 2 Mass ELISA and PIP 3 Mass ELISA kit (purchased from Echelon) was used to detect the effect of B591 on the production of PIP2 and PIP3 in HCT116 cells.
[0067] 2. Experimental results: B591 can inhibit the activity of PI3Kα, PI3Kβ, PI3Kγ and PI3Kδ, IC50 are: PI3Kα=1.30±0.27μmol / L; PI3Kβ=0.36±0.13μmol / L; PI3Kγ=0.11±0.07μmol / L; PI3Kδ = 1.58±0.16μmol / L( Figure 1B ). B591 also significantly inhibited PIP 3 / PIP 2 The ratio of PIP 2 to PIP 3 The conversion of B591 was significantly reduced, further proving that B591 directly targets PI3K ( Figure 1C ). In addition, with the increase of ATP concentration, the kinase inhibitory activity of B591 decreased, indicating that B591 is an ATP-competitive...
Embodiment 3
[0069] B591 significantly inhibits mTORC1 and mTORC2 signaling pathways in different tumor cells.
[0070] 1. Cell lines: Human lung cancer A549 cells, human colon cancer HCT116 cells, human rhabdomyosarcoma RD cells and human breast cancer MDA-MB-468 cells were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. Cells were placed at 37°C, 5% CO 2 A549, HCT116 and RD cells were cultured in RPMI-1640 medium containing 10% fetal bovine serum; MDA-MB-468 cells were cultured in DMEM medium containing 10% fetal bovine serum.
[0071] 2. Experimental method: A549, HCT116, RD and MDA-MB-468 cell suspensions in the logarithmic growth phase were inoculated in 6-well plates. After the cells adhered to the wall, the original culture medium was removed, and different concentrations of B591 were added to each well of the experimental group, and the same amount of culture medium without B591 was added to each well of the control group, and the cells were processed and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com